Research Article - (2023)Volume 14, Issue 6
Paradoxical Responses in Biologic Therapy for Psoriasis: Unraveling Mechanisms and Optimizing Treatment Strategies
Jingyan Kong1,
Minghui Zhao2,
Xiaoyu Ma2*,
Fan Yang1* and
Hongxiao Gao2
*Correspondence:
Xiaoyu Ma,
Department of Basic Teaching and Research in Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine,
Tianjin,
China,
Email:
Fan Yang,
Department of Chinese Medicine and Cosmetology, Tianjin University of Traditional Chinese Medicine, Tianjin,
China,
Email:
Author info »
Abstract
This review article comprehensively investigates the paradoxical reactions provoked by biologics in the treatment of
psoriasis, examining the role of chemokines, inflammatory mediators, oxidative stress responses, as well as genetic and
environmental factors. Biologics, whilst offering significant relief to psoriasis symptoms, can in some cases exacerbate
the condition through the induction of other chemokines and inflammatory mediators, or by disrupting intracellular
antioxidant mechanisms. Additionally, the presence of gene polymorphisms in psoriasis patients may influence the
sensitivity to biologics, with specific polymorphisms in the HLA-Cw6, IL23R, and IL12B genes possibly impacting the
reactivity to TNF-α and IL-23 inhibitors. Environmental factors, such as infections, lifestyle, and psychological stress,
can also affect the occurrence of paradoxical reactions, with smoking, alcohol consumption, and infections potentially
impacting the efficacy and safety of biologics. This review underscores the importance of a holistic understanding of
the role of these factors in paradoxical reactions for optimizing psoriasis treatment plans.
Keywords
Psoriasis; Biologics; Immune system; Paradoxical reactions; Treatment optimization
Introduction
Psoriasis is a common chronic skin disease, primarily
characterized by local skin redness, scales, and itching [1]. The
prevalence of psoriasis varies among different regions and
populations, but it maintains a certain level of prevalence
worldwide [2]. The etiology of psoriasis is not entirely
understood, but research indicates that it’s associated with
aberrant activation of the immune system, genetic factors, and
environmental influences [3]. Moreover, the incidence of
psoriasis can increase with age, and there are gender differences,
with varying rates between men and women [4].
Biologics refer to drugs with specific biological activities
produced through genetic engineering. They can interfere with
specific cytokines and signaling pathways in the immune system, thereby achieving therapeutic effects in psoriasis [5]. Currently,
biologics have become an important treatment choice, especially
for patients who are resistant or intolerant to traditional
therapies. The use of biologics can significantly improve patients’
symptoms and quality of life, reduce the area and severity of skin
lesions, and maintain long-term efficacy [6-8]. Despite the notable
efficacy of biologics in the treatment of psoriasis, paradoxical
reactions occur. For instance, the long-term use of biologics may
lead to the suppression of immune function, increasing the risk
of opportunistic infections [9,10]. Therefore, understanding the
mechanisms of paradoxical reactions in biologic treatment is
important for optimizing treatment plans and reducing treatment
risks. Current studies have found that altering the dosage and
administration of biologics, and the combined use of other drugs
may help reduce the occurrence of paradoxical reactions [7,11].
The aim of this review is to provide a comprehensive analysis
and summary of the importance and current status of biologic
treatment for psoriasis, as well as the mechanisms of paradoxical
reactions. Through this comprehensive analysis, we hope to
provide a reference for optimizing the biologic treatment of
psoriasis.
Literature Review
Common types of biologic agents used for treating
psoriasis
Biologics, innovative medications with broad applications in
various diseases, have proven significantly effective in the field of
dermatology, especially in treating psoriasis. Currently, the
biologics commonly used in dermatology for psoriasis treatment
internationally include:
Tumor Necrosis Factor (TNF: Inhibitors are a class of drugs
that counteract the activity of TNF. This class of biologics
includes Etanercept, Infliximab, Adalimumab, golimumab, and
certolizumab, among others. They inhibit TNF activity, reducing
inflammation and thus alleviating the symptoms of psoriasis
[12-18].
Cell inhibitors: Common drugs include Rituximab and
Omalizumab. These medications inhibit the activity of B cells,
reducing the production of inflammatory factors and
autoantigens, thus modulating the function of the immune
system and relieving the inflammatory response in psoriasis
[19-21].
IL-12/23 inhibitors: Such as Ustekinumab and Briakinumab,
primarily inhibit IL-12 and IL-23 to suppress the activation of
Th1 and Th17 cells, thus alleviating inflammation [22,23].
Interleukin-17A (IL-17A: Inhibitors mainly include
secukinumab, ixekizumab, and brodalumab. These drugs
moderate the immune system by inhibiting the function of
IL-17A, thereby reducing psoriasis symptoms and the
inflammatory response [24,25].
IL-1 antagonists: Include, but are not limited to, Anakinra,
Canakinumab, and Gevokizumab. These medications modulate
the function of the immune system, alleviating inflammation
and thus improving symptoms in psoriasis patients. Anakinra, a
human recombinant IL-1Ra antagonist, can inhibit IL-1α and IL-
β and has shown results in the treatment of Generalized Pustular
Psoriasis (GPP) and patients with IL-1 receptor antagonist
deficiency [26,27]. Canakinumab, an anti-IL-1β antibody, has
shown benefits in the treatment of GPP [28,30]. As a novel anti-
IL-1β antibody, Gevokizumab can also be used in the treatment
of GPP patients [31,32].
IL-23 inhibitors: These are drugs that inhibit the cytokine
IL-23, an important immune-mediated factor closely associated
with the pathogenesis of psoriasis. Several specific drugs have
been developed, including ustekinumab, guselkumab,
tildrakizumab, and risankizumb. By interfering with the
signaling pathway of IL-23 and inhibiting its activation effect on
the immune system, these drugs achieve their therapeutic effect
in psoriasis [22,33-38] (Figure 1).
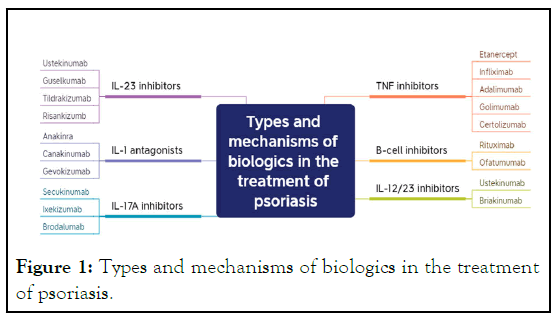
Figure 1: Types and mechanisms of biologics in the treatment
of psoriasis.
Mechanism of action and efficacy evaluation of
biologics
The mechanism of action of biologics in psoriasis treatment
primarily involves suppressing the inflammatory response,
blocking T-cell activation, and inhibiting B-cell function [13].
Regarding efficacy evaluation, the Psoriasis Area and Severity
Index (PASI) scoring is commonly employed to assess treatment
outcomes [39,40]. Research indicates that, in the treatment of
psoriasis, optimal results from biologics are generally achieved
after 4-6 weeks of continuous therapy [41,42]. Despite the
significant efficacy of biologics in psoriasis treatment, certain
adverse reactions are associated with their use. Common side
effects include infections [43], headaches [44], gastrointestinal
discomfort, and allergic reactions [45-47]. Moreover, some
biologics, such as Infliximab and Adalimumab, may pose a risk
of malignant tumors during use [48-51]. Therefore, regular
checks for complete blood count and liver and kidney function
assessments are necessary during the course of psoriasis
treatment with biologics to monitor potential adverse reactions.
The manifestation of paradoxical reactions in
psoriasis treatment with biologics
A paradoxical reaction refers to an unexpected response that
contradicts the theoretical mechanism of a drug during
treatment [52]. They can be categorized into mild, moderate,
and severe, based on their nature and intensity. Mild reactions
may only present as transient discomfort, moderate reactions
may lead to decreased treatment efficacy, and severe reactions
may threaten the patient’s life [53,54]. Biologics primarily work
by blocking the biological activity of inflammatory factors,
thereby suppressing inflammatory responses and alleviating
psoriatic lesions. However, paradoxical reactions can occur
during this treatment process, such as a worsening of psoriasis
or the emergence of new skin lesions, particularly common
when using anti-TNF drugs [55-57]. Here is a detailed overview
of various commonly used biologics:
Tumor Necrosis Factor (TNF) inhibitors: Infliximab,
Etanercept, and Adalimumab have been proven effective in
treating diseases such as psoriasis and psoriatic arthritis.
However, some studies have pointed out potential paradoxical
reactions associated with the use of TNF inhibitors, including
the emergence of new or worsening psoriatic rash;
gastrointestinal discomfort; increased risk of infections such as
tuberculosis, fungal infections, and bacterial infections; and potential disease rebound after discontinuation of TNF
inhibitors [46,58-62].
B cell inhibitors: Like Rituximab, primarily used to treat
autoimmune diseases such as rheumatoid arthritis and systemic
lupus erythematosus, it achieves disease control by selectively
eliminating CD20 positive B cells. However, some studies have
found that new or worsening psoriasis may occur during
treatment with B cell inhibitors, posing an increased risk of
certain infections such as fungal and bacterial infections, and
potential disease rebound after discontinuation [63-67].
IL-12/23 inhibitors: Like Ustekinumab, a biologic used to treat
moderate to severe psoriasis, it works by inhibiting the activation
and proliferation of Th1 and Th17 cells. However, some studies
have found paradoxical reactions may occur during treatment
with IL-12/23 inhibitors, such as atypical psoriasis responses like
collagenosis or psoriatic arthritis, increased risk of bacterial and
viral infections, and potential triggering or worsening of some
immune-mediated diseases like Crohn’s disease and ulcerative
colitis, as well as headaches, upper respiratory infections, and
skin irritation. [68-73].
IL-1 antagonists: Like Anakinra, used to treat some
inflammatory diseases, including psoriasis. However, some
studies have shown that paradoxical reactions may occur during
treatment with IL-1 antagonists, manifesting as rashes, including
psoriatic-like rash, reversible after discontinuation of the drug,
increased risk of bacterial and viral infections, and potential
triggering or worsening of some immune-mediated diseases like
Cohn’s disease and ulcerative colitis [74-78].
IL-23 inhibitors: IL-23 inhibitors achieve therapeutic effects by
suppressing immune responses, which could potentially suppress
the patient’s immune system, thereby increasing the risk of
infectious diseases [79]. Some studies have indicated that the
treatment of psoriasis with IL-23 inhibitors could lead to a
rebound of rash [80]. IL-23 inhibitors may also affect the
efficacy of vaccines [81-84].
IL-17A inhibitors: Some studies have found that IL-17A
inhibitors may lead to the occurrence of novel diseases during the
treatment of psoriasis, and the use of IL-17A inhibitors may
increase the risk of inflammatory bowel diseases (such as Cohn’s
disease and ulcerative colitis) [85]. Some research also indicates
that some patients may experience disease rebound, worsening
condition after discontinuation of IL-17A inhibitors [86].
Additionally, some patients may develop resistance to these drugs,
leading to a gradual decline in treatment efficacy (Figure 2).
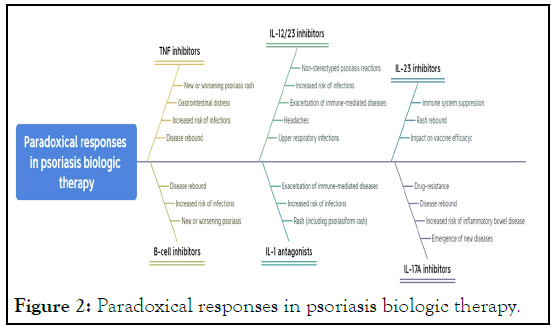
Figure 2: Paradoxical responses in psoriasis biologic therapy.
Impact of paradoxical reactions on treatment
efficacy and quality of life
Paradoxical reactions can potentially affect the treatment
outcome, prolonging or exacerbating the course of psoriasis,
thereby impacting patients’ quality of life. These reactions may
also instill fear and resistance towards the medication in
patients, lowering their adherence to the treatment, and thus
influencing the overall treatment efficacy. During the
administration of biologics, it is important to regularly conduct
safety assessments, closely monitor the emergence of paradoxical
reactions, and if severe reactions occur, the use of the drug
should be immediately discontinued and alternative treatment
options should be sought.
The treatment efficacy of biologics can be assessed through a
variety of indices, including the Psoriasis Area and Severity
Index (PASI), Patient Global Assessment (PGA) [87] and the
Health-Related Quality of Life (HRQoL) [88]. The degree of
improvement in these indices post-biologic treatment can reflect
the efficacy of the medication. Furthermore, long-term follow-up
of patients is necessary to monitor the durability of treatment
effects and the occurrence of paradoxical reactions. Hence,
paradoxical reactions in the treatment of psoriasis with biologics
not only concern the selection and usage of drugs but also
involve a comprehensive evaluation of treatment efficacy and
safety, requiring the joint attention and handling of both
physicians and patients.
Mechanisms underlying paradoxical reactions
The role and regulatory mechanisms of the immune system in
the treatment of psoriasis with biologics: The development and
progression of psoriasis are associated with immune system
abnormalities [89]. Biologics treat psoriasis by modulating the
immune system to suppress inflammatory responses. Studies have
shown that biologics can effectively control the development of
psoriasis by regulating the balance of cytokines such as Th1, Th2,
and Th17 [90,91], as well as by modulating the functions of
antigen presentation, phagocytosis, and natural killer cells
[92,93]. Additionally, biologics can activate anti-inflammatory
signaling pathways, such as NF-kB inhibitors and STAT3
inhibitors, to alleviate skin inflammation [94-97] (Figure 3).
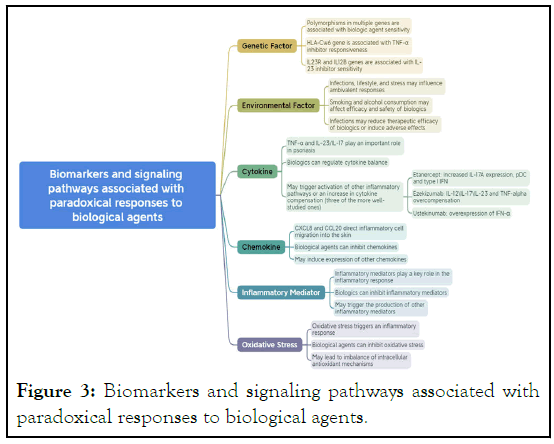
Figure 3: Biomarkers and signaling pathways associated with
paradoxical responses to biological agents.
Biomarkers and signaling pathways associated with
paradoxical reactions to biologics
Paradoxical reactions, characterized by a worsening or relapse of
symptoms after the use of biologics, is a common phenomenon
in the treatment of psoriasis [7,98,99]. The mechanisms
underlying these reactions are not fully understood but may be
related to the immunoregulatory effects of biologics, individual
differences, and environmental factors [100,101]. Certain
biomarkers and signaling pathways, such as cytokines,
chemokines, inflammatory mediators, and oxidative stress
responses, may play key roles in paradoxical reactions.
Monitoring these biomarkers and signaling pathway alterations
can help in early identification and prevention of paradoxical
reactions [100,102-104]. Psoriasis is a complex inflammatory
disease whose pathophysiology involves numerous signaling
pathways and cell types, including T cells, keratinocytes,
dendritic cells, and myriad cytokines like Tumor Necrosis Factor
(TNF) and Interleukins (ILs) [105-108]. Biologics target these
signaling pathways and cytokines to treat psoriasis, but
sometimes they may trigger paradoxical reactions.
Cytokines: Cytokines like TNF-α and the IL-23/IL-17 axis play
an important roles in psoriasis. Biologics such as TNF-α
inhibitors and IL-17 inhibitors can effectively alleviate symptoms.
However, some patients may experience worsening of their
condition after using these agents, possibly due to the activation
of other inflammatory pathways or compensatory increase in
cytokines [75,109-111].
Chemokines: Chemokines such as CXCL8 and CCL20 can
guide inflammatory cells to the skin, exacerbating
inflammation. Biologics can mitigate symptoms by inhibiting
these chemokines, but in some cases, they may induce the
expression of other chemokines, exacerbating the condition
[112-116].
Inflammatory mediators: Inflammatory mediators, including
various prostaglandins and leukotrienes, play a key role in
inflammatory responses. Biologics can alleviate symptoms by
inhibiting these mediators, but in some instances, they may
trigger the production of other inflammatory mediators,
worsening the condition [117-122].
Oxidative stress responses: Oxidative stress responses can trigger
inflammatory reactions and exacerbate skin lesions. Biologics
can improve the condition by inhibiting oxidative stress
responses, but in certain cases, they may disrupt the intracellular
antioxidant mechanisms, worsening the condition [123-126].
Genetic and environmental factors in paradoxical
reactions
Genetic and environmental factors play critical roles not only in
the pathogenesis of psoriasis but also in the occurrence of
paradoxical reactions. Studies have shown the presence of
polymorphisms in multiple genes in patients with psoriasis,
which may be related to the sensitivity of psoriasis to biologics
[127]. Polymorphism in the HLA-Cw6 gene has been found to be
associated with the reactivity of psoriasis to TNF-α inhibitors [128]. Another study found that polymorphisms in IL23R and
IL12B genes might influence the sensitivity of psoriasis patients
to IL-23 inhibitors [129]. Environmental factors such as
infections, lifestyle, and psychological stress could also affect the
occurrence of paradoxical reactions [130-132]. Smoking and
alcohol consumption could potentially impact the efficacy and
safety of biologics [133]. Furthermore, infections may lead to a
decrease in the therapeutic effect of biologics and even trigger
severe adverse reactions [134]. Therefore, a comprehensive
understanding of the role of genetic and environmental factors
in paradoxical reactions is vital for optimizing psoriasis
treatment plans.
Strategies for managing and preventing paradoxical
reactions
Firstly, for the identification and assessment of paradoxical
reactions, careful observation of symptom changes and adverse
reactions during the treatment process is necessary.
Simultaneously, a holistic evaluation should be conducted in
conjunction with individual differences and specific disease
characteristics. Necessary information could be gathered
through medical history inquiries, physical examinations, and
relevant laboratory tests to promptly identify and assess the
occurrence and severity of paradoxical reactions. Secondly,
various strategies can be employed in managing and treating
paradoxical reactions. On one hand, the drug dosage and
frequency of use could be reduced or adjusted to minimize the
occurrence and severity of adverse reactions [8,135,136]. On the
other hand, adjunctive treatment measures, such as local care,
physiotherapy, and traditional Chinese medicine, could be used
to alleviate adverse reactions and promote disease improvement
[137-140]. Lastly, for the prevention of paradoxical reactions and
personalized treatment strategies, a multi-faceted approach is
needed. Firstly, patients should be guided to adopt good lifestyle
habits, including regular sleep and meal schedules, balanced
diet, and moderate exercise, to enhance immune resistance
[141]. Psychological regulation is also vital to maintain a positive
mental and emotional state, which could be achieved through
psychological counseling and support. Moreover, personalized
treatment is of paramount importance. Treatment plans should
be customized based on the patient’s disease characteristics and
individual differences to achieve optimal therapeutic outcomes
[142].
Psoriasis is a chronic inflammatory skin condition characterized
primarily by patchy red skin covered with white scales. Patients
often experience symptoms such as itching and dryness, which
tend to worsen during the winter and lighten during the
summer [3].Currently, significant progress has been made in the
treatment of psoriasis using biologics. These biologics work by
modulating the immune system functions, reducing the
inflammatory response, and thus improving patient symptoms.
However, a number of paradoxical reactions may occur during
the biologic treatment of psoriasis, including immune
suppression, infection, and diminished vaccine response. The
mechanisms of biologic treatment primarily function by
inhibiting inflammatory mediators and regulating immune cell functions [143-145]. However, the specific mechanisms warrant
further exploration and research. In terms of future research
prospects and recommendations, current studies are primarily
focused on clinical observations and case studies. Future
research could delve deeper into the pathogenesis of psoriasis,
mechanisms of biologic therapy, and the occurrence
mechanisms of paradoxical reactions. Moreover, multi-center
and large-scale clinical studies can be conducted to more
comprehensively and objectively assess the efficacy and safety of
biologic treatments [146,147]. In conclusion, biologic treatment
for psoriasis is currently an effective therapeutic approach, albeit
potential paradoxical reactions may occur during the treatment
process. Future research can further explore the treatment
mechanisms and conduct more extensive and in-depth clinical
studies.
Conclusion
In conclusion, the management and prevention of paradoxical
reactions in psoriasis can be effectively addressed through
identification and assessment, management and treatment, as
well as prevention strategies and personalized treatment
approaches. The implementation of these strategies requires
considering the patient’s disease characteristics and individual
differences to enhance treatment outcomes and improve the
patients’ quality of life.
References
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA. 2020;323(19):1945-1960.
[Crossref] [Google Scholar] [PubMed]
- Parisi R, Iskandar IY, Kontopantelis E, Augustin M, Griffiths CE, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ. 2020;369.
[Crossref] [Google Scholar] [PubMed]
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021; 397(10281): 1301-1315.
[Crossref] [PubMed]
- Ding J, Joseph M, Chawla S, Yau N, Khosa Z, Khawaja F, et al. Disparities in psoriasis clinical trials: A cross-sectional analysis. J Am Acad Dermatol. 2022;87(6):1386-1389.
[Crossref] [Google Scholar] [PubMed]
- Xiao Q, Li X, Li Y, Wu Z, Xu C, Chen Z, et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. 2021;11(4):941-960.
[Crossref] [Google Scholar] [PubMed]
- Egeberg A, Ottosen MB, Gniadecki R, Broesby‐Olsen S, Dam TN, Bryld LE, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate‐to‐severe plaque psoriasis. Br J Dermatol. 2018;178(2):509-519.
[Crossref] [Google Scholar] [PubMed]
- Ortiz Álvarez J, Rodríguez Fernández-Freire L, Hernández-Rodríguez JC, Barabash-Neila R, Durán-Romero AJ, et al. Efficacy and safety of biological therapy in patients with psoriasis and recent neoplasia: Experience in real single-center clinical practice. Arch Dermatol Re. 2023;315(4):1045-1048.
[Crossref] [Google Scholar] [PubMed]
- Ribero S, Ortoncelli M, Mastorino L, Quaglino P, Dapavo P. Reduced doses of biological therapies in psoriasis: A new potentially “sustainable” frontier of psoriasis treatment. Expert Opin Biol Ther. 2023;23(9):867-868.
[Crossref] [Google Scholar] [PubMed]
- Murphy MJ, Cohen JM, Vesely MD, Damsky W. Paradoxical eruptions to targeted therapies in dermatology: A systematic review and analysis. J Am Acad Dermatol. 2022;86(5):1080-1091.
[Crossref] [Google Scholar] [PubMed]
- Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1-30.
[Crossref] [Google Scholar] [PubMed]
- Lozano CR. Safety of biological therapies: New data from BIOBADASER. Reumatol Clin. 2011;6:S1-S6.
[Crossref] [Google Scholar] [PubMed]
- Chédotal H, Narayanan D, Povlsen K, Gotfredsen CH, Brambilla R, Gajhede M, et al. Small-molecule modulators of tumor necrosis factor signaling. Drug Discov Today. 2023:103575.
[Crossref] [Google Scholar] [PubMed]
- Leone GM, Mangano K, Petralia MC, Nicoletti F, Fagone P. Past, present and (foreseeable) future of biological anti-tnf alpha therapy. J Clin Med. 2023;12(4):1630.
[Crossref] [Google Scholar] [PubMed]
- Ducharme E, Weinberg JM. Etanercept. Expert Opin Biol Ther. 2008;8(4):491-502.
[Crossref] [Google Scholar]
- Li S, Li G, Li X, Wu F, Li L. Etanercept ameliorates psoriasis progression through regulating high mobility group box 1 pathway. Skin Res Technol. 2023;29(4):e13329.
[Crossref] [Google Scholar] [PubMed]
- Kishimoto M, Komine M, Kamiya K, Sugai J, Kuwahara A, Mieno M, et al. Drug survival of tumor necrosis factor-alpha inhibitors and switched subsequent biologic agents in patients with psoriasis: A retrospective study. Dermatol Ther (Heidelb). 2023:1-4.
[Crossref] [Google Scholar] [PubMed]
- Onsun N, Güneş B, Kaya G, Güçkan Işık B, Yabacı Tak A. Comparison of survival and retention rates between infliximab and adalimumab for psoriasis: 10-year experience at a single tertiary center. Dermatology. 2023;239(3):355-361.
[Crossref] [Google Scholar] [PubMed]
- Hagino T, Saeki H, Kanda N. Biomarkers and predictive factors for treatment response to tumor necrosis factor- ff inhibitors in patients with psoriasis. J Clin Med, 2023, 12(3):974.
[Crossref] [PubMed]
- Fetter T, Niebel D, Braegelmann C, Wenzel J. Skin-associated B cells in the pathogenesis of cutaneous autoimmune diseases-implications for therapeutic approaches. Cells. 2020;9(12):2627.
[Crossref] [Google Scholar] [PubMed]
- Kim DW, Park SK, Woo SH, Yun SK, Kim HU, Park J. New-onset psoriasis induced by rituximab therapy for non-Hodgkin lymphoma in a child. Eur J Dermatol. 2016;26(2):190-191.
[Crossref] [Google Scholar] [PubMed]
- Abdelmaksoud A, Temiz SA, Daye M, Yavuz S, Wollina U, Lotti T, et al. Response of chronic refractory psoriasis vulgaris with urticaria to combined secukinumab and omalizumab: A case report and review of the literature. J Cosmet Dermatol. 2023;22(4):1416-1418.
[Crossref] [Google Scholar] [PubMed]
- Gönülal M, Balcı DD, Öztürk A, Doğan S. Effectiveness and safety of ustekinumab for the treatment of psoriasis; six years of clinical experience. J Dermatolog Treat. 2023;34(1):2241941.
[Crossref] [Google Scholar] [PubMed]
- Schurich A, Raine C, Morris V, Ciurtin C. The role of IL-12/23 in T cell-related chronic inflammation: Implications of immunodeficiency and therapeutic blockade. Rheumatology (Oxford). 2018;57(2):246-254.
[Crossref] [Google Scholar] [PubMed]
- Vsn M, Mothe RK, Hyderboini RK, Likhar N, Ganji K, Singuru S, et al. Ssystematic review and meta-analysis of briakinumab, a fully human interleukin 12/23 monoclonal antibody, for the treatment of moderate to severe chronic plaque psoriasis. Value in Health. 2016;19(3):A123.
[Crossref] [Google Scholar]
- Wang C, Torisu-Itakura H, Hanada T, Matsuo T, Cai Z, Osaga S, et al. Treatment persistence of interleukin-17 inhibitor class drugs among patients with psoriasis in Japan: A retrospective database study. J Dermatolog Treat. 2023;34(1):2229465.
[Crossref] [Google Scholar] [PubMed]
- Dastoli S, Passante M, Loconsole F, Mortato E, Balato A, Piccolo V, et al. Long-term efficacy and safety of secukinumab in real life: A 240 weeks multicenter study from Southern Italy. J Dermatolog Treat. 2023;34(1):2200868.
[Crossref] [Google Scholar] [PubMed]
- Naik HB, Pichard DC, Schwartz DM, O'Brien M, Masciocchi M, Thompson J, et al. Anakinra for refractory pustular psoriasis: A phase II, open-label, dose-escalation trial. J Am Acad Dermatol. 2022;87(6):1380-1383.
[Crossref] [Google Scholar] [PubMed]
- Macca L, Pomi FL, Ingrasciotta Y, Morrone P, Trifirò G, Guarneri C. Hidradenitis suppurativa and psoriasis: The odd couple. Front Med (Lausanne). 2023;10.
[Crossref] [Google Scholar] [PubMed]
- Skendros P, Papagoras C, Lefaki I, Giatromanolaki A, Kotsianidis I, Speletas M, et al. Successful response in a case of severe pustular psoriasis after interleukin1β inhibition. Br J Dermatol. 2017;176(1):212-215.
[Crossref] [Google Scholar] [PubMed]
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin‐1β inhibitor gevokizumab. Br J Dermatol. 2015;173(1):239-241.
[Crossref] [Google Scholar] [PubMed]
- Moreau A, Le Vée M, Jouan E, Denizot C, Parmentier Y, Fardel O. Effect of gevokizumab on interleukin-1β-mediated cytochrome P450 3A4 and drug transporter repression in cultured human hepatocytes. Eur J Drug Metab Pharmacokinet. 2017;42:871-878.
[Crossref] [Google Scholar] [PubMed]
- Kong L, huang DW, Lu JJ, Zhang Y, Li Y, Yi X, et al. Development of bullous pemphigoid during treatment of psoriasis with ustekinumab: A case report and literature review. Front Med (Lausanne). 2023;10 :1171802.
[Crossref] [PubMed]
- Bartholomew E, Chung BY, Davis M, Yeroushalmi S, Chung M, Hakimi M, et al. Rapid Remission of Sunburn-Induced Guttate Psoriasis with Guselkumab. Dermatol Ther (Heidelb). 2023:1-6.
[Crossref] [Google Scholar] [PubMed]
- Gerdes S, Hoffmann M, Asadullah K, Korge B, Mortazawi D, Krüger N, et al. Effectiveness, safety and quality of life effects of guselkumab and ustekinumab in patients with psoriasis: Week 104 results from the non-interventional, prospective, German multicentre PERSIST study. J Eur Acad Dermatol Venereol. 2023.
[Crossref] [Google Scholar] [PubMed]
- Egeberg A, Jullien D, Gaarn Du Jardin K, Thaçi D. Five-year safety of tildrakizumab in patients with moderate-to-severe psoriasis from two phase 3 trials (reSURFACE 1 and reSURFACE 2): Number needed to harm for occurrence of adverse events of special interest. J Dermatolog Treat. 2023;34(1):2220447.
[Crossref] [Google Scholar] [PubMed]
- Ruggiero A, Fabbrocicni G, Cacciapuoti S, Potestio L, Gallo L, Megna M. Tildrakizumab for the treatment of moderate-to-severe psoriasis: Results from 52 weeks real-life retrospective study. Clin Cosmet Investig Dermatol. 2023:529-536.
[Crossref] [Google Scholar] [PubMed]
- Huang K, Wu X, Li Y, Lv C, Yan Y, Wu Z, et al. Artificial intelligence–based psoriasis severity assessment: Real-world study and application. J Med Internet Res. 2023;25:e44932.
[Crossref] [Google Scholar] [PubMed]
- Maravilla-Herrera P, Merino M, Alfonso Zamora S, Balea Filgueiras J, Carrascosa Carrillo JM, Delgado Sánchez O, et al. The social value of a PASI 90 or PASI 100 response in patients with moderate-to-severe plaque psoriasis in Spain. Front Public Health. 2023;11:1000776.
[Crossref] [Google Scholar] [PubMed]
- Papp KA, Weinberg MA, Morris A, Reich K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: A multicentre, randomised, placebo-controlled, phase 2b study. Lancet. 2021;397(10284):1564-1575.
[Crossref] [Google Scholar] [PubMed]
- Puig L, Bakulev AL, Kokhan MM, Samtsov AV, Khairutdinov VR, Morozova MA, et al. Efficacy and safety of netakimab, a novel anti-IL-17 monoclonal antibody, in patients with moderate to severe plaque psoriasis. Results of a 54-week randomized double-blind placebo-controlled PLANETA clinical trial. Dermatol Ther (Heidelb). 2021;11(4):1319-1332.
[Crossref] [Google Scholar] [PubMed]
- Gambra MP, Montesinos JJ, Valenzuela MT, Cárcamo ME. Risk of infections associated with the use of biological medications; a review. Rev Med Chil. 2020;148(8):1155-1170.
[Crossref] [Google Scholar] [PubMed]
- Carrascosa JM, Del-Alcazar E. Apremilast for psoriasis treatment. G Ital Dermatol Venereol. 2020;155(4): 421-433.
[Crossref] [PubMed]
- AbuHilal MD, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: A retrospective study. Cutan Med Surg. 2016;20(4):313-316.
[Crossref] [Google Scholar] [PubMed]
- Jiraskova Zakostelska Z, Reiss Z, Tlaskalova-Hogenova H, Rob F. Paradoxical reactions to anti-TNFα and anti-IL-17 treatment in psoriasis patients: Are skin and/or gut microbiota involved?. Dermatol Ther. 2023;13(4):911-933.
[Crossref] [Google Scholar]
- Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Ther. 2019;32(2):e12817.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Friedman M, Liu G, Deodhar A, Chu CQ. Do tumor necrosis factor inhibitors increase cancer risk in patients with chronic immune-mediated inflammatory disorders?. Cytokine. 2018;101:78-88.
[Crossref] [Google Scholar] [PubMed]
- Fuxench ZC, Shin DB, Beatty AO, Gelfand JM. The risk of cancer in patients with psoriasis: A population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152(3):282-290.
[Crossref] [Google Scholar] [PubMed]
- Langley RG, Strober BE, Gu Y, Rozzo SJ, Okun MM. Benefit‐risk assessment of tumour necrosis factor antagonists in the treatment of psoriasis. Br J Dermatol. 2010;162(6):1349-1358.
[Crossref] [Google Scholar] [PubMed]
- Semaka A, Salopek TG. Risk of developing melanoma with systemic agents used to treat psoriasis: A review of the literature. J Cutan Med Surg. 2022;26(1):87-92.
[Crossref] [Google Scholar] [PubMed]
- Miyagawa F. Pathogenesis of paradoxical reactions associated with targeted biologic agents for inflammatory skin diseases. Biomedicines. 2022;10(7):1485.
[Crossref] [Google Scholar] [PubMed]
- Kremenevski I, Sander O, Sticherling M, Raithel M. Paradoxical reactions to biologicals in chronic inflammatory systemic diseases. Dtsch Arztebl Int. 2022;119(6):88.
[Crossref] [Google Scholar] [PubMed]
- Yagiz B, Lermi N, Coskun BN, Dalkilic E, Kiraz S, Erden A, et al. The predictors of paradoxical reactions, especially psoriasis, to biologic therapy—findings from the TReasure database: A 5-year follow-up study. Rheumatology. 2023:kead318.
[Crossref] [Google Scholar] [PubMed]
- Bataille P, Layese R, Claudepierre P, Paris N, Dubiel J, Amiot A, et al. Paradoxical reactions and biologic agents: A French cohort study of 9303 patients. Br J Dermatol. 2022;187(5):676-683.
[Crossref] [Google Scholar] [PubMed]
- Ancor SG, Reolid A, Fisas LH, Munoz-Aceituno E, Llamas-Velasco M, Sahuquillo-Torralba A, et al. DNA copy number variation associated with anti-tumour necrosis factor drug response and paradoxical psoriasiform reactions in patients with moderate-to-severe psoriasis. Acta Derm Venereol. 2021;101(5).
[Crossref] [Google Scholar] [PubMed]
- Su HJ, Chan YP, Shen PC, Ku CL, Ng CY. Anti-IL-17A antibody-associated de novo vitiligo: Case report and review of literature. Frontiers in Immunology. 2023;13:1077681.
[Crossref] [Google Scholar] [PubMed]
- Kim HW, Kim EH, Lee M, Jung I, Ahn SS. Risk of cancer, tuberculosis and serious infections in patients with ankylosing spondylitis, psoriatic arthritis and psoriasis treated with IL-17 and TNF-α inhibitors: A nationwide nested case-control analysis. Clin Exp Rheumatol. 2023;41:1491-1499.
[Crossref] [Google Scholar] [PubMed]
- Li M, You R, Su Y, Zhou H, Gong S. Characteristic analysis of adverse reactions of five anti-TNFɑ agents: A descriptive analysis from WHO-VigiAccess. Front Pharmacol. 2023;14.
[Crossref] [Google Scholar] [PubMed]
- Pauline O, Robert M, Bernardeau C, Hlavaty A, Fusaroli M, Roustit M, et al. Assessment of reported adverse events after interchanging between tnf-α inhibitor biosimilars in the who pharmacovigilance database. BioDrugs. 2023:1-9.
[Crossref] [Google Scholar] [PubMed]
- Zhou X, Mubanga D, Chen Z, Bi X. Secondary generalized cutaneous mucinosis developed during etanercept treatment in a patient with psoriasis. Clin Cosmet Investig Dermatol. 2022:987-992.
[Crossref] [Google Scholar] [PubMed]
- Coşkunol İ, Turan O, Baysak A, Solmaz D, Can G. Frequency of latent tuberculosis in patients receiving anti-TNF-alpha therapy. Afr Health Sci. 2023;23(2):128-132.
[Crossref] [Google Scholar]
- Berger JR, Houff SA, Major EO. Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs. 2009;1(6):583-589.
[Crossref] [Google Scholar] [PubMed]
- Fagni F, Simon D, Tascilar K, Schoenau V, Sticherling M, Neurath MF, et al. COVID-19 and immune-mediated inflammatory diseases: Effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3(10):e724-e736.
[Crossref] [Google Scholar] [PubMed]
- Kovarik J. From immunosuppression to immunomodulation: Current principles and future strategies. Pathobiology. 2013;80(6):275-281.
[Crossref] [Google Scholar] [PubMed]
- Guidelli GM, Fioravanti A, Rubegni P, Feci L. Induced psoriasis after rituximab therapy for rheumatoid arthritis: A case report and review of the literature. Rheumatol Int. 2013;33:2927-2930.
[Crossref] [Google Scholar] [PubMed]
- Seror R, Sordet C, Guillevin L, Hachulla E, Masson C, Ittah M, et al. Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjögren’s syndrome. Ann Rheum Dis. 2007;66(3):351-357.
[Crossref] [Google Scholar] [PubMed]
- Puzenat E, Bronsard V, Prey S, Gourraud PA, Aractingi S, Bagot M, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24:10-16.
[Crossref] [Google Scholar] [PubMed]
- Reich K, Langley RG, Papp KA, Ortonne JP, Unnebrink K, Kaul M, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365(17):1586-1596.
[Crossref] [Google Scholar] [PubMed]
- Patel BM, Ramos Rivers C, Koutroumpakis F, Ahsan M, Dueker J, Hashash J, et al. Ustekinumab-induced remission of two cases of refractory cutaneous crohn’s disease. Inflammatory Bowel Diseases. 2021;27(10):e124.
[Crossref] [Google Scholar] [PubMed]
- Toussirot É, Michel F, Béreau M, Binda D. Ustekinumab in chronic immune-mediated diseases: A review of long term safety and patient improvement. Patient preference and adherence. 2013:369-377.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Qin G, Meng Z, Pei D. Body mass index, disease duration and tumor necrosis factor inhibitor history predict reduced ustekinumab response in Chinese psoriasis patients: A real-world study. Indian J Dermato. 2021;66(5):574.
[Crossref] [Google Scholar] [PubMed]
- Davidson L, van den Reek JM, van Hunsel F, de Jong EM, Kullberg BJ. Global risk of bacterial skin infections and herpesviridae infections with ustekinumab, secukinumab, and tumour necrosis factor-alpha inhibitors: Spontaneous reports of adverse drug reactions from the world health organization pharmacovigilance center. Acta Dermato-Venereologica. 2022;102.
[Crossref] [Google Scholar] [PubMed]
- López GMA, Taboada MVM, Vela GMC, Llaca FH, Bernal VJF. New onset psoriasis following treatment with the interleukin1 receptor antagonist anakinra. Br J Dermatol. 2008;158(5):1146-1148.
[Crossref] [Google Scholar] [PubMed]
- Karamanakos A, Vergou T, Panopoulos S, Tektonidou MG, Stratigos AJ, Sfikakis PP. Psoriasis as an adverse reaction to biologic agents beyond anti-TNF-α therapy. Eur J Dermatol. 2021;31(3):307-317.
[Crossref] [Google Scholar] [PubMed]
- Tauber M, Viguier M, Alimova E, Petit A, Lioté F, Smahi A, et al. Partial clinical response to anakinra in severe palmoplantar pustular psoriasis. Br J Dermatol. 2014;171(3):646-649.
[Crossref] [Google Scholar] [PubMed]
- Viguier M, Guigue P, Pagès C, Smahi A, Bachelez H. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: Lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153(1):66-67.
[Crossref] [Google Scholar] [PubMed]
- Fleischmann RM, Tesser J, Schiff MH, Schechtman J, Burmester GR, Bennett R, et al. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(8):1006-1012.
[Crossref] [Google Scholar] [PubMed]
- Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo-and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418-431.
[Crossref] [Google Scholar] [PubMed]
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): Results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650-661.
[Crossref] [Google Scholar] [PubMed]
- Abdo AI, Tye GJ. Interleukin 23 and autoimmune diseases: Current and possible future therapies. Inflamm Res. 2020;69(5):463-480.
[Crossref] [Google Scholar] [PubMed]
- Nogueira M, Warren RB, Torres T. Risk of tuberculosis reactivation with interleukin (IL)‐17 and IL‐23 inhibitors in psoriasis–time for a paradigm change. J Eur Acad Dermatol Venereol. 2021;35(4):824-834.
[Crossref] [Google Scholar] [PubMed]
- Palakornkitti P, Nimmannitya K, Rattanakaemakorn P. Biological therapy in Psoriasis: An emphasis on its dermatologic adverse events. Asian Pac J Allergy Immunol. 2021;39(4):215-230.
[Crossref] [Google Scholar] [PubMed]
- Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75(4):687-695.
[Crossref] [Google Scholar] [PubMed]
- Mehta P, Lawrence A, Aggarwal A. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis.2023;82(6):e152.
[Crossref] [Google Scholar] [PubMed]
- Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400-409.
[Crossref] [Google Scholar] [PubMed]
- Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician's global assessment. J Am Acad Dermatol. 2004;51(4):563-569.
[Crossref] [Google Scholar] [PubMed]
- Kasiem FR, Kok MR, Luime JJ, Tchetverikov I, Wervers K, Korswagen LAet al. The burden of psoriasis in patients with early psoriatic arthritis. Rheumatology. 2022;61(4):1570-1578.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Xu X, Cheng H, Zhou F. AIM2 and Psoriasis. Front Immunol. 2023;14:1085448.
[Crossref] [Google Scholar] [PubMed]
- Park J, Son MJ, Ho CC, Lee SH, Kim Y, An J, et al. Transcriptional inhibition of STAT1 functions in the nucleus alleviates Th1 and Th17 cell-mediated inflammatory diseases. Front Immunol. 2022;13:1054472.
[Crossref] [Google Scholar] [PubMed]
- Yan K, Zhang F, Ren J, Huang Q, Yawalkar N, Han L. MicroRNA-125a-5p regulates the effect of Tregs on Th1 and Th17 through targeting ETS-1/STAT3 in psoriasis. J Transl Med. 2023;21(1):678.
[Crossref] [Google Scholar] [PubMed]
- Babaie F, Omraninava M, Gorabi AM, Khosrojerdi A, Aslani S, Yazdchi A, et al. Etiopathogenesis of psoriasis from genetic perspective: An updated review. Curr Genomics. 2022;23(3):163.
[Crossref] [Google Scholar] [PubMed]
- Marusina AI, Ji-Xu A, Le ST, Toussi A, Tsoi LC, Li Q, et al. Cell-Specific and Variant-Linked Alterations in Expression of ERAP1, ERAP2, and LNPEP Aminopeptidases in Psoriasis. J Invest Dermatol. 2023;143(7):1157-1167.
[Crossref] [Google Scholar] [PubMed]
- Jia HY, Qiu HY, Zhang MD, Hou JJ, Zhou ML, Wu Y. Lenalidomide attenuates IMQ-induced inflammation in a mouse model of psoriasis. Biomed Pharmacother. 2022;156:113883.
[Crossref] [Google Scholar] [PubMed]
- Srivastava AK, Srivastava S, Kumar V, Ghosh S, Yadav S, Malik R, et al. Identification and mechanistic exploration of structural and conformational dynamics of NF-kB inhibitors: Rationale insights from in silico and in vitro studies. J Biomol Struct Dyn. 2023:1-21.
[Crossref] [Google Scholar] [PubMed]
- Lu H, Gong H, Du J, Gao W, Xu J, Cai X, et al. Piperine ameliorates psoriatic skin inflammation by inhibiting the phosphorylation of STAT3. Int Immunopharmacol. 2023;119:110221.
[Crossref] [Google Scholar] [PubMed]
- Ravipati A, Nolan S, Alphonse M, Dikeman D, Youn C, Wang Y, et al. IL-6R/signal transducer and activator of transcription 3 signaling in keratinocytes rather than in T cells induces psoriasis-like dermatitis in mice. J Invest Dermatol. 2022;142(4):1126-1135.
[Crossref] [Google Scholar] [PubMed]
- Camela E, Potestio L, Fabbrocini G, Megna M. Paradoxical reactions to biologicals for psoriasis. Expert Opin Biol Ther. 2022;22(12):1435-1437.
[Crossref] [Google Scholar] [PubMed]
- Fisher S, Ziv M. Skin and soft tissue infections in biological therapy for psoriasis-A case report and systematic review of the literature. Int J Dermatol. 2021;60(11):1429-1434.
[Crossref] [Google Scholar] [PubMed]
- Krušič M, Jezernik G, Potočnik U. Gene ontology analysis highlights biological processes influencing responsiveness to biological therapy in psoriasis. Pharmaceutics. 2023;15(8):2024.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Koppu S, Perche PO, Feldman SR. The cytokine mediated molecular pathophysiology of psoriasis and its clinical implications. Int J Mol Sci. 2021;22(23):12793.
[Crossref] [Google Scholar] [PubMed]
- Ceccarelli M, Rullo VE, Berretta M, Cacopardo B, Pellicano GF, et al. New generation biologics for the treatment of psoriasis and psoriatic arthritis. State of the art and considerations about the risk of infection. Dermatol Ther. 2021;34(1):e14660.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Li C, Li H, Yu H, Zhang X, Yan M, et al. Identification of a TH2‐high psoriasis cluster based on skin biomarker analysis in a Chinese psoriasis population. J Eur Acad Dermatol Venereol. 2021;35(1):150-158.
[Crossref] [Google Scholar] [PubMed]
- Solberg SM, Aarebrot AK, Sarkar I, Petrovic A, Sandvik LF, Bergum B, et al. Mass cytometry analysis of blood immune cells from psoriasis patients on biological therapy. Eur J Immunol. 2021;51(3):694-6702.
[Crossref] [Google Scholar] [PubMed]
- Contassot E, French LE. Killing two birds with one stone: TNF antagonists downregulate systemic IL-1β in Psoriasis. J Invest Dermatol. 2021;141(3):476-478.
[Crossref] [Google Scholar] [PubMed]
- Xiang R, Hu L, Li S, Wei Z, Song Z, Chen Z, et al. Tiamulin inhibits TNF-α and alleviates psoriasis-like dermatitis. J Dermatol Sci. 2022;107(1):32-40.
[Crossref] [Google Scholar] [PubMed]
- Avallone G, Maronese CA, Murgia G, Carrera CG, Mastorino L, Roccuzzo G, et al. Interleukin-17 vs. Interleukin-23 Inhibitors in Pustular and Erythrodermic Psoriasis: A retrospective, multicentre cohort study. J Clin Med. 2023;12(4):1662.
[Crossref] [Google Scholar] [PubMed]
- Zhu C, Ren Y, Yao H, Feng B, Liu L, Zheng M. Heparanase contributes to psoriatic lesions through crosstalk with IL-17 pathway. Indian J Dermatol. 2023;68(1):59-66.
[Crossref] [Google Scholar] [PubMed]
- Carrasquillo OY, Pabón-Cartagena G, Barrera-Llaurador J, Colón-Fontanez F, Martín-García RF. Psoriatic alopecia in a patient with Crohn Disease: An uncommon manifestation of tumor necrosis factor α inhibitors. Cutis. 2021;107(4):E48-E55.
[Crossref] [Google Scholar] [PubMed]
- Ho CH, Silva AA, Tomita B, Weng HY, Ho IC. Differential impacts of TNFα inhibitors on the transcriptome of Th cells. Arthritis Res Ther. 2021;23:1-3.
[Crossref] [Google Scholar] [PubMed]
- Ożóg MK, Grabarek BO, Wierzbik-Strońska M, Świder M. Neurological complications of biological treatment of psoriasis. Life. 2022;12(1):118.
[Crossref] [Google Scholar] [PubMed]
- Choudhary S, Anand R, Pradhan D, Bastia B, Kumar SN, Singh H, et al. Transcriptomic landscaping of core genes and pathways of mild and severe psoriasis vulgaris. Int J Mol Med. 2021;47(1):219-231.
[Crossref] [Google Scholar] [PubMed]
- Furue K, Ito T, Tanaka Y, Yumine A, Hashimoto-Hachiya A, Takemura M, et al. Cyto/chemokine profile of in vitro scratched keratinocyte model: Implications of significant upregulation of CCL20, CXCL8 and IL36G in Koebner phenomenon. J Dermatol Sci. 2019;94(1):244-251.
[Crossref] [Google Scholar] [PubMed]
- Kanda N, Hau CS, Tada Y, Tatsuta A, Sato S, Watanabe S. Visfatin enhances CXCL8, CXCL10, and CCL20 production in human keratinocytes. Endocrinology. 2011;152(8):3155-3164.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Xie S, Jiang W, Tang S, Shi Y. Discovering novel biomarkers associated with the pathogenesis of psoriasis: Evidence from bioinformatic analysis. Int J Gen Med. 2022:2817-2833.
[Crossref] [Google Scholar] [PubMed]
- Abdallah BH, Seeler S, Bregnhøj A, Ghatnekar G, Kristensen LS, Iversen L. et al. Heat shock protein 90 inhibitor RGRN-305 potently attenuates skin inflammation. Front Immunol. 2023;14:1128897.
[Crossref] [Google Scholar] [PubMed]
- Gomes L, Santos A, Gil Â, Filipe P, Quinhones-Levy P, Bicho M. 309 interleukin-1β, cyclooxygenase-2 and prostaglandin E2 receptor 4 genetic polymorphisms may modulate inflammation influencing the severity of psoriasis. J Invest Dermatol. 2022;142(12):S233.
[Google Scholar]
- Morin S, Tremblay A, Dumais E, Julien P, Flamand N, Pouliot R. Eicosapentaenoic acid influences the lipid profile of an in vitro psoriatic skin model produced with T cells. Biomolecules. 2023;13(9):1413.
[Crossref] [Google Scholar] [PubMed]
- Wadi JS, Dunya AD, Jabir M, Najim MA, Jawad SF, Hamzah SS, et al. Exploring the interaction between 3-D structure of TLR 9 and prostaglandin analogues. Arab J Chem. 2023;16(5):104692.
[Crossref] [Google Scholar]
- Paine A, Brookes PS, Bhattacharya S, Li D, de La Luz Garcia Hernandez M, Tausk F, et al. Dysregulation of bile acids, lipids, and nucleotides in psoriatic arthritis revealed by unbiased profiling of serum metabolites. Arthritis Rheumatol. 2023;75(1):53-63.
[Crossref] [Google Scholar] [PubMed]
- Tsukayama I, Kawakami Y, Tamenobu A, Toda K, Maruoka S, Nagasaki Y, et al. Malabaricone C derived from nutmeg inhibits arachidonate 5-lipoxygenase activity and ameliorates psoriasis-like skin inflammation in mice. Free Radic Biol Med. 2022;193(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Yokomizo T, Shimizu T. The leukotriene B4 receptors BLT1 and BLT2 as potential therapeutic targets. Immunol Rev. 2023;317(1):30-41.
[Crossref] [Google Scholar] [PubMed]
- Blagov A, Sukhorukov V, Guo S, Zhang D, Eremin I, Orekhov A. The role of oxidative stress in the induction and development of psoriasis. Front Biosci. 2023;28(6):118.
[Crossref] [Google Scholar]
- Dobrică EC, Cozma MA, Găman MA, Voiculescu VM, Găman AM. The involvement of oxidative stress in psoriasis: A systematic review. Antioxidants. 2022;11(2):282.
[Crossref] [Google Scholar] [PubMed]
- Salahuddin Z, Rafi A, Muhammad H, Aftab U, Akhtar T, Zafar MS, et al. Revolutionalizing the age old conventional treatment of psoriasis: An animal based comparative study between methylprednisolone and different doses of a novel anti-oxidant humanin analogue (HNG). Int Immunopharmacol. 2022;110:108990.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Tang S, Jiang Y, Long F, He F, Liu J, et al. Oxidative stress induces meiotic defects of oocytes in a mouse psoriasis model. Cell Death Dis. 2022;13(5):474.
[Crossref] [Google Scholar] [PubMed]
- Al-Janabi A, Eyre S, Foulkes AC, Khan AR, Dand N, Burova E, et al. Atopic polygenic risk score is associated with paradoxical eczema developing in patients with psoriasis treated with biologics. J Invest Dermatol. 2023;143(8):1470-1478.
[Crossref] [Google Scholar] [PubMed]
- Talamonti M, Botti E, Galluzzo M, Teoli M, Spallone G, Bavetta M, et al. Pharmacogenetics of psoriasis: HLA Cw6 but not LCE3B/3C deletion nor TNFAIP3 polymorphism predisposes to clinical response to interleukin 12/23 blocker ustekinumab. Br J Dermatol. 2013;169(2):458-463. [Crossref]
[Google Scholar] [PubMed]
- Mahil SK, Capon F, Barker JN. Genetics of psoriasis. Dermatol Clin. 2015;33(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Naik PP. Stem cell therapy as a potential treatment option for psoriasis. An Bras Dermatol. 2022;97(4):471-477.
[Crossref] [Google Scholar] [PubMed]
- Nardis C. Latent virus reactivation risk and biological drugs: Chronic inflammatory and immune-mediated disorders. Int J Immunopathol Pharmacol. 2013;26(4):983-989.
[Crossref] [Google Scholar] [PubMed]
- Singh A, Easwari TS. Recent advances in psoriasis therapy: Trends and future prospects. Current Drug Targets. 2021;22(15):1760-1771.
[Crossref] [Google Scholar] [PubMed]
- Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: A systematic review and meta-analysis. Br J Dermatol. 2014;170(2):304-314.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Xu P, Fan W, Yang G, Wang J, Cheng Q, et al. Risk of infection in patients with spondyloarthritis and ankylosing spondylitis receiving antitumor necrosis factor therapy: A meta-analysis of randomized controlled trials. Exp Ther Med. 2017;14(4):3491-3500.
[Crossref] [Google Scholar] [PubMed]
- Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072.
[Crossref] [Google Scholar] [PubMed]
- Diotallevi F, Paolinelli M, Radi G, Offidani A. Latest combination therapies in psoriasis: Narrative review of the literature. Dermatol Ther. 2022;35(10):e15759.
[Crossref] [Google Scholar] [PubMed]
- Hodak E, Gottlieb AB, Segal T, Politi Y, Maron L, Sulkes J, et al. Climatotherapy at the Dead Sea is a remittive therapy for psoriasis: Combined effects on epidermal and immunologic activation. J Am Acad Dermatol. 2003;49(3):451-457.
[Crossref] [Google Scholar] [PubMed]
- Perrotta FM, Scriffignano S, Benfaremo D, Ronga M, Luchetti MM, Lubrano E. New insights in physical therapy and rehabilitation in psoriatic arthritis: A review. Rheumatol Ther. 2021;8(2):639-649.
[Crossref] [Google Scholar] [PubMed]
- Yang XY, Cai WL, Guo CL, Chen QH. Chinese medicine as supporting therapy for psoriasis: Past, present, and future. Chin J Integr Med. 2023;29(3):280-288.
[Crossref] [Google Scholar] [PubMed]
- Ma X, Li D, Zhao M, He J, Yang F, Kong J. Bloodletting cupping combined with conventional measures therapy for psoriasis: A systematic review and meta-analysis of randomized controlled trials. Front Med. 2023;10:1132928.
[Crossref] [Google Scholar] [PubMed]
- Jensen P, Zachariae C, Christensen R, Geiker NR, Schaadt BK, Stender S, et al. Effect of weight loss on the severity of psoriasis: A randomized clinical study. JAMA Dermatol. 2013;149(7):795-801.
[Crossref] [Google Scholar] [PubMed]
- Kunchok A, Aksamit AJ, Davis JM, Kantarci OH, Keegan BM, Pittock SJ, et al. Association between tumor necrosis factor inhibitor exposure and inflammatory central nervous system events. JAMA Neurol. 2020;77(8):937-946.
[Crossref] [Google Scholar] [PubMed]
- Singla S, Putman M, Liew J, Gordon K. Association between biological immunotherapy for psoriasis and time to incident inflammatory arthritis: A retrospective cohort study. Lancet Rheumatol. 2023;5(4):e200-e207.
[Crossref] [Google Scholar]
- Singla S, Putman M, Liew J, Gordon K. Differentiating biologics to prevent psoriatic arthritis in patients with psoriasis-Authors' reply. Lancet Rheumatol. 2023;5(6):e313.
[Crossref] [Google Scholar]
- Thorley J. Biological therapy for psoriasis and development of psoriatic arthritis. Lancet. 2021; 3(11): E755-E.
[Google Scholar]
- Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (Be ready): A multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475-486.
[Crossref] [Google Scholar] [PubMed]
- Papp K, Warren RB, Green L, Reich K, Langley RG, Paul C, et al. Safety and efficacy of mirikizumab versus secukinumab and placebo in the treatment of moderate-to-severe plaque psoriasis (OASIS-2): A phase 3, multicentre, randomised, double-blind study. Lancet Rheumatol. 2023;5(9):e542-E552.
[Crossref] [Google Scholar]
- Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487-498.
[Crossref] [Google Scholar] [PubMed]
- Tehlirian C, Peeva E, Kieras E, Scaramozza M, Roberts ES, Singh RS, et al. Safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of the oral TYK2 inhibitor PF-06826647 in participants with plaque psoriasis: A phase 1, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Rheumatol. 2021;3(3):e204-e213.
[Crossref] [Google Scholar]
Author Info
Jingyan Kong1,
Minghui Zhao2,
Xiaoyu Ma2*,
Fan Yang1* and
Hongxiao Gao2
1Department of Chinese Medicine and Cosmetology, Tianjin University of Traditional Chinese Medicine, Tianjin, China
2Department of Basic Teaching and Research in Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine,
Tianjin, China
Citation: Kong J, Zhao M, Ma X, Yang F, Gao H (2023) Paradoxical Responses in Biologic Therapy for Psoriasis: Unraveling Mechanisms and
Optimizing Treatment Strategies. J Clin Exp Dermatol Res. 14:651.
Received: 23-Oct-2023, Manuscript No. JCEDR-23-27970;
Editor assigned: 25-Oct-2023, Pre QC No. JCEDR-23-27970 (PQ);
Reviewed: 10-Nov-2023, QC No. JCEDR-23-27970;
Revised: 17-Nov-2023, Manuscript No. JCEDR-23-27970 (R);
Published:
24-Nov-2023
, DOI: 10.35841/2329-9509.23.14.651
Copyright: © 2023 Kong J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
