
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Review Article - (2021)
Paraquat is a quaternary compound used as an herbicide for destroying weed and is highly toxic for humans. Its ingestion leads to multi-organ dysfunction, leading to liver insufficiency and lung fibrosis, which are life-threatening due to respiratory failure. A Pubmed, EMBASE, Ovid, and Cochrane library search was done, all authors reviewed all literature and relevant studies, and data were included in references. Paraquat is available in commercial 20% concentrate form, as 2.5% granules, and 0.2% aerosol. Mortality rates are as high as 65% in patients who ingest concentrated formulation compared to 4% in those who ingest diluted solution (25% w/v). Paraquat undergoes redox-cycling to generate reactive oxygen species leads to its toxic effects. This highly reactive oxygen and nitrite species result in multi-organ toxicity which is maximally seen in the lungs. Absorption occurs primarily through the small intestine. Peak concentration in plasma and maintenance of plasma paraquat levels are the two main factors that decide its concentration in the lungs. The destructive phase is followed by the proliferative phase, in which there is the presence of extensive fibrosis. High dose intake leads to acute respiratory distress syndrome, myocardial necrosis, cerebral edema, and renal failure, which leads to multi-organ failure. The diagnosis of paraquat poisoning is based on history. Simple bedside methods like urine or plasma dithionate tests are used to assess systemic paraquat toxicity. Management stands on four pillars which are reducing absorption, supportive care, antioxidant therapy, and immunosuppression.
Paraquat poisoning; Toxicity; Antioxidant therapy
Paraquat, also known as methyl viologen, is a quaternary compound whose properties were first described in 1993 by Michales and Hill [1]. It was introduced as an herbicidal agent into the market in August 1962 by Imperial Chemical Industries, now Syngenta [2,3]. Paraquat is registered and used in over 120 countries worldwide due to its low cost, easy availability, and excellent herbicidal properties for destroying weed, and it is highly toxic for humans. Its ingestion leads to multi-organ dysfunction, leading to liver insufficiency and lung fibrosis, which are life-threatening due to respiratory failure [4]. For agriculture use, Paraquat is available in 10%-30% concentration, broadly neutral but can also be irritating and corrosive. It contains an aliphatic detergent that enhances its entry into the cells, enhancing its toxicity. Commercially paraquat is sold in 20% concentrate, 2.5% granules or 0.2% aerosol [4,5]. Mortality rates are as high as 65% in patients who ingest concentrated formulation compared to 4% in those ingest diluted solution (25% w/v) [6]. Paraquat formulation is dark-blue or green in color to distinguish it from common beverages and contains a powerful stanching and emetic agent. Paraquat ingestion causes a cyclic reduction and oxidation reaction producing reactive oxygen species and depletes Nicotinamide Adenine Nucleotide Phosphate (NADPH) [7]. Paraquat is taken up against the concentration gradient in the lungs, thus leading to inflammation involving leucocyte recruitment and late pulmonary fibrosis, causing hypoxemia resistant to the treatment. Patients ingesting 40 ml of 24% concentrate paraquat develop fulminant poisoning and die within hours to days due to multi-organ failure. As little as 16 ml of paraquat ingestion can cause moderate to severe poisoning leading to pulmonary fibrosis and severe hypoxemia within 1-2 weeks [8-10]. It has a high case fatality rate attributed to its inherent toxicity and lack of effective treatment [11]. Due to its high toxicity to humans, the European Union, withdrew Paraquat from its market in 2007.
Paraquat’s ability to undergo redox-cycling to generate reactive oxygen species leads to its toxic effects. It is metabolized to PQ+, which reoxidizes to PQ2+ inside the cell with the help of NADHUbiquinone oxidoreductase, NADPH cytochrome P450 reductase, xanthine oxidase, and nitric oxide synthase enzyme system [12-19]. This process generates superoxide, which leads to the formation of peroxynitrite (ONOO-) and hydroxyl free radicle (HO-). This highly reactive oxygen and nitrite species result in multi-organ toxicity, the maximum seen in the lungs [20]. Electrophilic free radicles generated by this process cause compromise to cell membrane function and trigger apoptosis via lipid peroxidation (Figure 1) [21,22]. In the mitochondria, Paraquat is reduced by NADH-Ubiquinone Oxidoreductase, which leads to superoxide formation [23,24]. Permeability of the inner mitochondrial membrane is increased, which is calciumdependent and leads to membrane depolarization, uncoupling, and matrix swelling, causing mitochondrial toxicity. Redox cycling rapidly oxidizes NADPH, decreasing glutathione production, causing impaired defense against oxidative stress. Reactive oxygen species activate the Nuclear Factor Kappa β (NF-Κβ). After activation, it induces target genes involved in inflammation and leads to inflammatory enzymes, cytokines, and chemokines. This results in platelet aggregation, Fibrinogenesis, and activation of inflammatory cells [25-27]. Paraquat, thus leads to apoptosis of cells via reactive oxygen species, NF-Κβ, and peroxynitrite species [28-31]. Major organs targeted by herbicidal poisoning are the lungs, proximal convoluted tubules in the kidneys, and the liver's rough and smooth endoplasmic retinaculum [32-37].
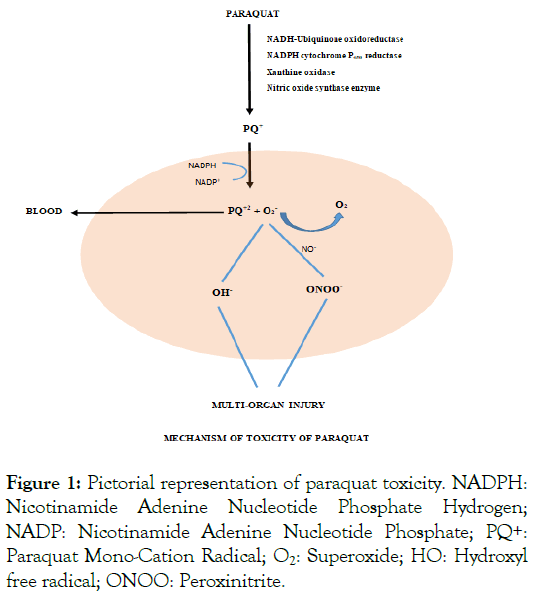
Figure 1: Pictorial representation of paraquat toxicity. NADPH: Nicotinamide Adenine Nucleotide Phosphate Hydrogen; NADP: Nicotinamide Adenine Nucleotide Phosphate; PQ+: Paraquat Mono-Cation Radical; O2: Superoxide; HO: Hydroxyl free radical; ONOO: Peroxinitrite.
Paraquat, a highly water-soluble compound, causes almost all poisonings due to the ingestion of the herbicide. Absorption occurs primarily through the small intestine, up to 5% in 1-6 hours. It is rapidly reabsorbed via a carrier-mediated transport system for choline on the brush border membrane of the small intestine, though the net fraction absorbed is relatively low [38,39]. Concurrent food ingestion decreases the amount of systemic reabsorption. Paraquat may be detected in urine as early as 1 hour after the ingestion. The peak plasma concentration is attained within 4 hours after ingestion [40,41]. Which remains relatively constant for 30 hours as demonstrated in the animal models [42]. During this period, the concentration in the lung rises several times the plasma concentration. Peak concentration in plasma and maintenance of plasma paraquat levels are the two main factors determining its concentration in the lungs. The pharmacokinetics of Paraquat differs substantially in humans as compared to animal models. Plasma paraquat levels decline rapidly after the first 15 hours of the half-life due to tissue distribution. Thus, any interventions, like administration of adsorbents, should be done within the first few hours or preferably minutes from the herbicidal ingestion [43].
Ocular exposure causes corrosive injury with local ulceration and scarring; however, it does not lead to systemic toxicity. Inhalation in occupation or agricultural settings can only cause nasal and tracheobronchial mucosal irritation as well as corrosion. The distribution of Paraquat in plasma is best explained by a three compartment system in which blood is considered the central compartment. The second compartment is composed of highly perfused tissues such as the liver, heart, kidney. Rapid exchanges occur between blood and this compartment. The third compartment comprises lung tissue, primarily type 1 and 2 pneumocytes and Clara cells. Exchanges between blood and lungs are typically slow. Half-life (T 1/2) of Paraquat in the lung is much higher than T 1/2 in other organs [44]. Provided renal functions are normal, peak concentration in the lung is achieved after 5-7 hours of ingestion [45]. Renal failure impairs the excretion of Paraquat by its usual route. It has been proved that impairment of renal function by 5% leads to a 5-fold higher concentration in plasma [46]. Most of the orally administered Paraquat is excreted unchanged in the urine, and only a tiny fraction is metabolized. The absorbed fraction is almost completely excreted unchanged through kidneys, nearly 80%-90% in 1st 6 hours, whereas almost 100% of the absorbed drug is excreted within 24 hours [47]. Paraquat excretion occurs both by glomerular filtration as well as tubular secretion.
Pathophysiology of Paraquat to cause multi-organ dysfunction progresses in two stages. The first stage leads to acute damage of highly perfused organs such as kidneys, liver, heart, and lungs. Death can occur during this period, if Paraquat is ingested in large amounts and is associated with pulmonary, circulatory, and renal failure. The second stage exclusively involves pulmonary damage. Paraquat is selectively accumulated in the lungs involving a polyamine uptake system [48,49]. Classically; two distinct phases are described in the development of pulmonary lesions, which coincide with early and late clinical stages. The first phase, known as the destructive phase, destroys type 1 alveolar and type 2 epithelial cells. The speed at which damage develops depends on the dose taken and route of administration. The earliest observed microscopic changes occur in type 1 alveolar epithelial cells, including cell swelling and increases in the content of mitochondria and ribosomes [50,51]. These changes are suggestive of increased metabolic activity. It ultimately leads to the rupture of the cell, exposing the basement membrane. Because type 1 alveolar epithelial cells are mainly involved in the exchange of gases between alveolar space and capillaries, Paraquat compromises lung function from the beginning only. Damage to type 2 cells occurs slightly later than type 1 cells. They play a major role in surfactant formation. Type 2 cells also have a role in defense against toxic agents as they are rich in NADPH cytochrome P450 reductase and undergo mitotic division and replace type 1 damaged cells. Damage to type 2 epithelial cells leads to increased surface tension within the alveolus, which draws the fluid from capillaries to produce edema [52]. Abnormalities in endothelial cells adjacent to damaged alveolar epithelial cells include fragmentation, vacuolization, and widened intercellular junctions [53-55] are detected after 72-96 hours of herbicide ingestion. The destructive phase is followed by the proliferative phase, in which there is the presence of extensive fibrosis, described as a compensatory repair mechanism to damaged alveolar epithelial cells. If the dose taken is high, resulting alveolitis is usually more severe and widespread. The earliest morphologic indicator is the appearance of profibroblasts. These cells undergo differentiation to form mature fibroblasts, which ultimately lay down collagen and ground substance to produce fibrosis [56,57] Ultimately, the normal architecture of the lung is destroyed, thereby reducing gas exchange, which can lead to death due to the presence of severe hypoxia. Paraquat, when exposed to kidneys, develops large vacuolation in the proximal convoluted tubules leading to necrosis. Congestion and hepatocellular injury are associated with rough and smooth endoplasmic reticulum degranulation, and mitochondrial damage occurs in the liver. The corrosive effects of Paraquat lead to necrosis of skin or the mucosal membranes, causing oral, oesophageal, or gastric ulceration, leading to perforation.
Immediately after ingestion, patients complain of buccopharyngeal, oesophageal, and epigastric pain. The tongue is swollen with characteristic ulcerations (paraquat tongue). Oesophageal and gastric ulceration are noted, which eventually leads to perforation. Poisoning by Paraquat is categorized into mild, moderate to severe and fulminant toxicity [58-61]. Ingestion of Paraquat of less than 20 mg/ kg produces mild symptoms, including nausea, vomiting, and diarrhea, and usually does not cause hepatic, renal, or pulmonary manifestations. More than 20 mg/kg but less than 50 mg/kg of Paraquat causes significant pulmonary fibrosis. Renal failure and hepatocellular necrosis develop on the second to fifth day post-exposure. Hepatotoxicity is evident by elevated liver enzymes, jaundice. Hepatic Injury is usually mild to moderate and leads to centrilobular hepatocellular necrosis and cholestasis [59]. A dose of more than 50 mg/kg is typically fatal within few hours of ingestion. Acute respiratory distress syndrome, myocardial necrosis, cerebral edema, and renal failure lead to multi-organ failure [62-64]. Pulmonary edema with progressive respiratory failure, bloody diarrhea convulsions, and circulatory failure also develops.
The immediate diagnosis of acute paraquat poisoning is based on the history and is primarily clinical. History of toxin ingestion, amount consumed, and characteristic features like pulmonary or renal involvement with circulatory failure may lead to clinical suspicion of paraquat intake. Simple bedside methods like urine or plasma dithionate tests are used to assess systemic paraquat toxicity. Dithionate, in alkaline medium, reduces Paraquat to blue radical (if urine paraquat is more than 1 mg/dl or plasma paraquat 2 gm/dl), giving a qualitative assessment of paraquat poisoning. Daily, routine laboratory investigations like renal function tests, liver functions, complete blood count, arterial blood gas analysis, and serum electrolytes should be done. A simple chest radiograph may detect pulmonary fibrosis, acute respiratory distress syndrome, pneumomediastinum, or pneumothorax [65]. Severity Index of Paraquat Poisoning (SIPP score) is the best predictor of survival after self-poisoning. It is calculated by multiplying the time from paraquat ingestion and serum paraquat levels at admission. Its calculation can accurately predict prognosis and survival estimates [66,67].
Due to its high case fatality rate, patients with paraquat toxicity either due to ingestion (accidental or suicidal) or intense dermal exposure require prompt hospitalization and experimental therapy. The prognosis and severity of paraquat poisoning can be predicted using history and specific laboratory investigations. Formulation of Paraquat, concentration and the amount of Paraquat ingested, time since ingestion, and presence of food in the gut helps assess the mortality. Spontaneous emesis and early gastric decontamination decrease the mortality rate as it decreases the absorption of Paraquat in the system. Suicidal poisoning carries a poor outcome as the amount of Paraquat ingested is much more significant than accidental ingestion (mild intoxication with 20 mg/Kg, moderate with 20-50 mg/kg, and severe toxicity with more than 50 mg/kg paraquat concentration) [68]. A recent meal before the ingestion delays the absorption and thus leads to a favorable outcome. Formation of esophageal or gastric ulceration within 24 hours of ingestion of Paraquat is lethal. Endoscopic examination within the first 24 hours gives an accurate assessment and thus helps in predicting mortality [60]. Depth of ulcerations is an indirect measure of the amount and concentration of Paraquat consumed and absorbed systemically. Early-onset renal failure (within 24 hours of consumption) carries a grave prognosis compared to the patients with preserved renal function [59,69-71]. Measuring the plasma and urine concentration of Paraquat are the most reliable methods to predict the outcome of paraquat poisoning as the severity and the rate of toxicity is dose-dependent [72]. A color change to dark blue in urinary paraquat detection is associated with a mortality rate of 100% [73,74]. Plasma parquet concentration can be obtained by five normograms and formulae, which was initially presented by Proudfoot [75-78]. Patients complaining of burning sensation over the skin after dermal paraquat exposure carry a poorer prognosis. In clinical practice and presentation, paraquat poisoning is almost always fatal; however, the exact mortality rate or outcome is yet to be established.
All paraquat poisoning requires immediate treatment and monitoring in a hospital setting as the window of opportunity is very narrow. The main goal of the treatment is to remove Paraquat from the GIT (preventing its absorption), increase its excretion from blood, and prevent pulmonary toxicity with antiinflammatory agents and some newer therapies.
Preventing absorption
Early decontamination to limit exposure is the most crucial step in the successful treatment of paraquat poisoning. Following dermal exposure, all the clothes should be removed immediately, and the skin should be washed gently with soap and water to prevent transdermal absorption. Specific care should be taken to avoid harsh scrubbing as skin abrasions increase transdermal absorption. Ophthalmic exposure is managed by rinsing the eyes with tepid water or normal saline for 15-20 minutes. Whole gut lavage, oral administration of mineral adsorbent, or induction of emesis play a pivotal role in management in acute settings. Airway patency, breathing, and circulation is to be maintained. Gastric lavage should not be used without an adsorbent. Fuller’s earth and bentonite, along with activated charcoal, are agents of choice for gastric decontamination [79-81]. Activated charcoal (2 g/Kg body weight, maximum up to 100 grams) is administered unless contraindicated (protracted vomiting, severe burns of oral mucosa). A total of 3 doses at two-hourly intervals can be administered [1,82]. As Paraquat achieves peak concentration very early, decontamination is helpful if done within 1-2 hours of ingestion. Charcoal Hemoperfusion (CHP) enhances the extracorporeal elimination of Paraquat, thus preventing its uptake by the various organs. Plasma paraquat levels of ≤ 3 mg/L may benefit from charcoal hemoperfusion [83].
Supportive therapy
Intravenous fluids and electrolytes are substituted to tackle dehydration caused due to Paraquat. As the kidney is the primary route of paraquat excretion, hemodialysis may be required in acute kidney injury. As oxygen provides an additional substrate for the formation of free radicals, hypooxygenation is suggested unless the PaO2 falls below 40 mm/Hg [4,84]. It can be achieved by artificial ventilation using FiO2<21, High positive end-expiratory pressure, and continuous positive pressure ventilation.
Specific therapy
To date, there has been no specific antidote or treatment for paraquat poisoning. However, various treatment modalities are tried and are currently experimental. There are no widely accepted treatment guidelines or good quality evidence for the treatment of paraquat poisoning.
Superoxide dismutase or related enzymes, when used in animal models, resulted in a reduction in mortality [1,85]. However, when tried in patients with paraquat toxicity, it failed to ameliorate the toxic effects of the herbicide [86]. Vitamin C, Vitamin E, and desferoxime are also not helpful in preventing lung damage in paraquat toxicity. In animal models, clofibrate is protective against herbicide-induced pulmonary toxicity and mortality when given before paraquat administration; however, no human studies support this effect.1 N-Acetyl cysteine, a cell membrane precursor of glutathione (GSH), acts as a free radicle scavenger [87,88]. There are few cases of paraquat poisoning being successfully treated using N-Acetyl cysteine in the treatment cocktail [1,89-91]. Despite showing potential, some treatment modalities like selenium, niacin, riboflavin plus vitamin C, and Angiotensin-Converting Enzyme Inhibitors (ACE) require human studies to prove their benefit [1,92].
The most widely proclaimed treatment approach to paraquat poisoning is immunosuppression. It is postulated that inhibition of the acute inflammatory response may protect against lung fibrosis and death. Cyclophosphamide (1 gram for two days), methylprednisolone (1 gram for three days), and dexamethasone (20 mg per day for 14 days) are the most widely used agents in paraquat poisoning [11,68,93,94]. Many human studies using a pulse of cyclophosphamide, methylprednisolone, and dexamethasone have shown positive results; However, these studies are not randomized controlled, involve a small population and have a bias in their data analysis, and so are criticized [1,95]. Until proper and adequately powered randomized controlled trials confirm their benefits; immunosuppression treatment is considered experimental.
Radiotherapy is successful in reversing the effects of Paraquat by preventing fibroblast proliferation but does not help reduce the mortality associated with the herbicide ingestion [96].
Edaravone, an antiapoptotic, antineurotic, and antiinflammatory compound, has free radicle scavenging properties. It is considered beneficial for preventing oxidative stress to the kidneys and liver, however it does not reduce pulmonary fibrosis [67-95]. Lung transplant is not helpful in Paraquat-associated pulmonary fibrosis, as most of the Paraquat is accumulated in the muscles; Thus, during the weaning process, the herbicide is released from muscles, resulting in new-onset pulmonary fibrosis.
Easy availability, rapid and severe toxicity, and no specific antidote make Paraquat a lethal toxin use for suicidal intent in Low, Medium income countries. Currently, there is no specific effective treatment targeting this herbicidal poisoning; however, antioxidants and immunosuppression is a new ray of hope in trying to revert the toxic effects of Paraquat and prevent mortality. Some human studies have shown positive results, but large multicentric controlled trials are required to establish efficacy and treatment protocols.
Citation: Navneet A, Wadhera S, Dhibar PD (2021) Paraquat Poisoning: ‘What we do and do not know.’ J Clin Toxicol. S19:004.
Received: 09-Sep-2021 Accepted: 23-Sep-2021 Published: 30-Sep-2021 , DOI: 10.35248/2161-0495.21.s19.004
Copyright: © 2021 Navneet A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.