Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research - (2023)Volume 12, Issue 12
Introduction: This study examined the association of parity, age at first childbirth, and age at last childbirth with premature menopause, hysterectomy, and bilateral oophorectomy before age 40 in women in the United States.
Material and methods: The data stemmed from the National Health and Nutrition Examination Survey spanning from 1999 to 2018, with 13,108 women over 40 included. The exposure variables were parity, age at first childbirth, and age at last childbirth. The outcome variable was premature menopause, hysterectomy, and bilateral oophorectomy before the age of 40. Logistic regression models were utilized to estimate unadjusted and adjusted odds ratios (ORs) (95% confidence intervals [CIs]) controlling for confounders in the occurrence of premature menopause, hysterectomy, or bilateral oophorectomy before turning 40. We also used logistic regression models with restricted cubic splines to depict the association between parity and age at childbirth, treated as continuous variables, and the outcome variables.
Results: Parity was not associated with premature menopause, but nulliparity and parity of one had lower odds of having a hysterectomy before age 40, with adjusted ORs (95% CI) of 0.41 (0.31-0.54) and 0.81 (0.66-0.99) compared to parity of two. Nulliparity was also linked to a lower likelihood of having bilateral oophorectomy before age 40 (OR=0.58; 95% CI:0.39-0.88). Age at first childbirth was negatively related to hysterectomy or bilateral oophorectomy before 40 but not premature menopause. In comparison to age at first childbirth of 25-29 years, the adjusted ORs (95% CIs) for hysterectomy risks in women with age at first delivery of <20, 20-24, 30+ years were 2.08 (1.52-2.85), 1.40 (1.06-1.86), and 0.46 (0.23-0.91), respectively. The corresponding adjusted ORs (95% CIs) for bilateral oophorectomy risks were 2.53 (1.51-4.24), 1.66 (1.04-2.63), and 0.70 (0.26-1.84), respectively. Besides, age at last childbirth also presented inverse and nonlinear associations with premature menopause, hysterectomy, or bilateral oophorectomy prior to age 40.
Conclusions: Nulliparity was associated with lower risks of hysterectomy or bilateral oophorectomy before age 40, while early first childbirth age was related to the increased risks. Late age at last childbirth was linked to reduced risks of premature menopause, hysterectomy, or bilateral oophorectomy before age 40.
Parity, Childbirth, Women, Nulliparity, Premature Menopause, Hysterectomy, Oophorectomy
Menopause represents the last stage of female reproductive aging, signifying the end of menstruation and fertility [1]. Women who experienced premature menopause before the age of 40 had increased risks of cardiovascular disease, type 2 diabetes, osteoporosis, cognitive impairments, and potentially premature death [2-8]. Premature menopause is typically described as the cessation of ovulation prior to the age of 40, often resulting from primary ovarian insufficiency [1]. Bilateral oophorectomy is a surgical surgery in which both ovaries are removed, whereas a hysterectomy entails the removal of the uterus [9]. Undergoing hysterectomy or bilateral oophorectomy prior to reaching the typical age of natural menopause might potentially result in enduring adverse health consequences, including impacts on hormonal fluctuations, cardiovascular health, and bone health [10- 12].
Multiple factors, including genetic predisposition, environmental exposures, lifestyle behaviors, and reproductive characteristics, can contribute to premature menopause, hysterectomy, or oophorectomy in women [13]. Previous studies have examined the relationship between parity, age at first childbirth, age at last childbirth, and age at natural menopause, but the overall evidence remains inconclusive [14-18]. Some population-based studies suggested that parity was associated with age at natural menopause [18-22], while other studies failed to identify any significant association between parity and age at natural menopause [23-25].
There is limited research exploring the relationship between parity and premature menopause, hysterectomy, or bilateral oophorectomy, and the association of age at first childbirth and age at last childbirth with the outcomes of interest. Although several studies examining reproductive factors related to hysterectomy involved parity and age at childbirth [23, 26, 27], few studies have explored the association between childbirth and hysterectomy or bilateral oophorectomy before age 40. Therefore, we utilized the National Health and Nutrition Examination Survey (NHANES) data to investigate the association of parity, age at first childbirth, and age at last childbirth with premature menopause, hysterectomy, or bilateral oophorectomy prior to turning 40 in women in the United States.
Study Population
NHANES is a cross-sectional health survey conducted on a representative sample of individuals that adequately reflects the nation, with unweighted response rates of approximately 70% [28]. The survey used a multi-stage probability sampling method, stratified by demographic groups, to select American civilians who were not institutionalized. We obtained the database from publicly available files.
Data analysis was conducted on ten 2-year survey cycles of NHANES from 1999 to 2018, focusing on women over 40. Individuals with a history of cancer or missing pregnancy data were excluded. Nulliparous women whose age at menopause was below the 95th percentile (32 years) for age at first childbirth in this study were also excluded to reduce premature menopause resulting in nulliparity. Ultimately, the present analysis included 13,108 women, comprising 1,418 nulliparous and 11,690 parous women.
Outcome and Exposure Variables
The outcome variables included premature menopause, hysterectomy before age 40, and bilateral oophorectomy before age 40. Participants who met the following criteria were classified as having premature menopause: absence of menstruation for at least of 12 months and experiencing menstrual cessation prior to the age of 40, excluding cases related to pregnancy, breastfeeding, hysterectomy, or oophorectomy. If a woman underwent a hysterectomy prior to the age of 40, it was classified as a hysterectomy before age 40. Likewise, the term bilateral oophorectomy before the age of 40 denoted the surgical removal of both ovaries before to reaching the age of 40.
The exposure variables comprised parity, age at first childbirth, and age at last childbirth. Parity referred to the number of live births and was determined by responses to several questions, including inquiries about previous pregnancies and the total number of live births. If participants had no live births, their parity value was 0. For women who gave birth at least once, the number of live births was categorized as 1, 2, 3, 4, or 5+ [29]. Age at first and last childbirth was established based on responses regarding age at first and last live birth.
Potential Confounders
The potential confounding variables included age, race, marital status, education, family monthly poverty level index (FMPLI), smoking, age at menarche, birth control usage, and the number of miscarriages/abortions/stillbirths [30-32]. Age, FMPLI, and age at menarche were continuous variables. Race was classified into non-Hispanic White, Black, Hispanic, and other races/ ethnicities. Education level was stratified into high school or below and college/university or above. Marital status was classified as married/cohabiting, widowed/divorced, or never married.
We determined smoking status by asking if participants had smoked at least 100 cigarettes in their lifetime and if they currently smoke. Information on birth control pill use was obtained by asking if they had ever taken them. The number of miscarriages/ abortions/stillbirths was calculated as the difference between total pregnancies and live births.
To address missing covariate values, we used multiple imputations based on observed data assuming they were missing completely at random. We utilized the "mice" package in R software to deal with missing values [33-36].
Statistical Analysis
We adhered to NHANES analysis principles for merging data from 1999-2018 and using sample weights [37]. We used mean ± standard deviation for continuous variables and frequency (weighted proportions) for categorical ones to describe participant characteristics. The Rao-Scott Chi-square test was utilized to compare weighted proportions among different parity levels, while ANOVA was employed to compare the mean values of continuous variables in multiple groups.
We performed multivariable logistic regression with survey weights to estimate odds ratios (ORs) values with 95% confidence intervals (CIs). Both unadjusted and adjusted models were used to examine the association between the exposures and the outcomes. For 13,108 participants (including nulliparous and parous women), we investigated the association between parity and premature menopause, hysterectomy, or bilateral oophorectomy before age 40. Among the 11,690 parous women, we further explored the association of parity and age at first and last childbirth with premature menopause, hysterectomy, or bilateral oophorectomy prior to age 40. We also used logistic regression models with restricted cubic splines to depict the association of parity and age at childbirth, treated as continuous variables, with the outcome variables.
To fully understand the relationship between the exposures, the outcomes, and potential effect modifiers, we examined how parity and age at childbirth interacted with age, race, education, marital status, smoking, birth control usage, and pregnancy loss, as well as the interactions among the exposure variables. We executed sensitivity analyses by excluding nulliparous women whose menopause age was below the 75th percentile (25 years) or the 90th percentile (29 years) for age at first childbirth. Additionally, we performed the sensitivity analysis on the complete observation database without any missing covariate values.
All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R software (version 4.3.0). Statistical significance was evaluated by a two-tailed P-value of less than 0.05.
The mean age of 13,108 participants was 59.4±12.6 years, ranging from 40 to 80. Race, marital status, and education varied significantly by parity level; nulliparous women were more likely to be White, unmarried, and possess a college education or higher than other parity categories. FMPLI mean decreased significantly with increasing parity. The mean menarche age was higher in women with 5+ parity than those with lower parity. Significant differences in smoking, birth control usage, and pregnancy loss were observed across parity levels. Among 11,690 parous women, the mean age at first and last childbirth was 22.1±4.8 years and 30.0±6.2 years, respectively, with significant variations across different parity groups [Table 1].
| Characteristics | Parity | P- value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5+ | ||
| N | 1,418 | 1,720 | 3,636 | 2,832 | 1,599 | 2,091 | |
| Age (years) a | 57.5±12.2 | 56.6±11.5 | 57.2±11.9 | 58.4±12.3 | 60.6±12.6 | 64.7±11.6 | <.0001 |
| Race b | <.0001 | ||||||
| White | 692(76.1) | 755(71.5) | 1,792(75.6) | 1,271(72.1) | 624(65.5) | 514(52.5) | |
| Black | 311(10.2) | 486(13.2) | 753(9.6) | 568(10.6) | 334(13.4) | 429(16.8) | |
| Hispanic | 262(7.1) | 279(7.5) | 747(8.8) | 789(12.0) | 538(14.9) | 858(23.4) | |
| Other | 153(6.5) | 200(7.7) | 344(6.1) | 204(5.3) | 103(6.2) | 102(6.3) | |
| Marital Status b | <.0001 | ||||||
| Married/living with a partner | 611(48.6) | 861(58.9) | 2,193(69.1) | 1,677(66.2) | 847(60.4) | 885(51.2) | |
| Widowed/divorced/separated | 384(23.6) | 658(33.6) | 1,276(28.1) | 1,041(31.6) | 677(36.5) | 945(45.2) | |
| Never married | 423(27.8) | 201(7.5) | 166(2.7) | 114(2.3) | 75(3.2) | 73(3.5) | |
| Education b | <.0001 | ||||||
| ≤High school | 511(28.8) | 687(35.2) | 1,563(36.9) | 1,530(44.9) | 1,028(55.0) | 1,519(71.6) | |
| ≥College | 907(71.2) | 1,033(64.8) | 2,073(63.1) | 1,302(55.1) | 571(45.0) | 384(28.4) | |
| Family monthly poverty level index a | 3.0±1.6 | 2.8±1.6 | 2.9±1.6 | 2.5±1.5 | 2.1±1.3 | 1.8±1.2 | <.0001 |
| Smoking b | 0.0007 | ||||||
| Current | 211(15.4) | 318(19.6) | 607(17.0) | 435(16.0) | 265(17.6) | 259(15.6) | |
| Former | 333(27.5) | 437(26.5) | 801(23.7) | 642(24.0) | 309(20.7) | 408(21.8) | |
| Never | 874(57.1) | 965(54.0) | 2,228(59.3) | 1,755(60.0) | 1,025(61.8) | 1,236(62.6) | |
| Age at menarche (years) a | 12.7±1.7 | 12.8±1.8 | 12.8±1.7 | 12.8±1.8 | 12.9±1.8 | 13.0±1.7 | <.0001 |
| Number of miscarriages/abortions/stillbirths b | <.0001 | ||||||
| 0 | 969(68.0) | 895(53.9) | 2,104(60.6) | 1,639(58.6) | 901(56.5) | 1,046(54.9) | |
| 1 | 235(19.1) | 450(24.7) | 872(23.4) | 702(25.4) | 417(25.1) | 434(23.3) | |
| ≥2 | 214(12.9) | 375(21.4) | 660(16.0) | 491(16.0) | 281(18.4) | 423(21.8) | |
| Ever taken birth control pills b | 798(66.5) | 1,157(77.6) | 2,592(79.6) | 1,842(71.1) | 969(63.9) | 941(52.6) | <.0001 |
| Age at first childbirth (years) b | <.0001 | ||||||
| <20 | ---- | 62(3.4) | 763(18.5) | 911(29.6) | 686(39.6) | 1,084(53.4) | |
| 20-24 | ---- | 1,435(81.4) | 1,401(37.5) | 1,239(42.9) | 696(43.3) | 673(39.0) | |
| 25-29 | ---- | 84(5.0) | 968(27.7) | 526(21.0) | 172(13.1) | 125(6.8) | |
| ≥30 | ---- | 139(10.2) | 504(16.3) | 156(6.5) | 45(4.0) | 21(0.8) | |
| Means+SDs a | ---- | 26.9±6.8 | 23.8±5.0 | 21.8±4.1 | 20.6±3.7 | 19.4±3.3 | <.0001 |
| Age at last childbirth (years) b | <.0001 | ||||||
| <25 | ---- | 671(36.0) | 953(24.4) | 453(16.0) | 176(10.5) | 76(4.1) | |
| 25-29 | ---- | 475(28.9) | 1,206(34.7) | 885(33.0) | 455(27.7) | 357(19.4) | |
| 30-34 | ---- | 313(18.9) | 906(26.0) | 861(28.6) | 492(31.2) | 541(30.9) | |
| ≥35 | ---- | 261(16.2) | 571(14.9) | 633(22.4) | 476(30.6) | 929(45.6) | |
| Means+SDs a | ---- | 26.9±6.8 | 28.5±5.5 | 30.1±5.3 | 31.2±5.4 | 34.4±5.6 | <.0001 |
Table 1: Characteristics of 13,108 women over the age of 40 according to different parity levels in the NHANES 1999-2018
Association between parity and premature menopause, hysterectomy, or bilateral oophorectomy before age 40
Among 13,108 nulliparous and parous women, there were 354 cases of premature menopause, 1,653 hysterectomies before age 40, and 624 bilateral oophorectomies before age 40. After adjusting for age, race, education, marital status, FMPLI, smoking, menarche age, the number of miscarriages/abortions/stillbirths, and birth control usage, there was an insignificant association between parity and premature menopause [Table 2].
However, parity was associated with hysterectomy or bilateral oophorectomy before age 40. The adjusted ORs (95% CI) for hysterectomy prior to age 40 with parity of 0, 1, 3, 4, and 5+ were 0.41 (0.31-0.54), 0.81 (0.66-0.99), 1.04 (0.84-1.29), 1.03 (0.80- 1.32), and 0.77 (0.60-1.03), respectively, compared to parity of 2. The adjusted ORs (95% CI) in the corresponding order for bilateral oophorectomy before tuning 40 were 0.58 (0.39-0.88), 0.97 (0.70-1.37), 1.29 (0.97-1.71), 0.78 (0.54-1.13), and 0.70 (0.47- 1.03), respectively [Table 2].
| Exposure | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Parity | Premature menopause (n=354) | ||
| 0 | 1.02(0.61-1.70) | 0.96(0.57-1.62) | 0.90(0.54-1.52) |
| 1 | 1.63(1.03-2.56) | 1.59(0.98-2.57) | 1.60(0.99-2.58) |
| 2 | 1 | 1 | 1 |
| 3 | 1.17(0.66-2.08) | 1.08(0.60-1.95) | 1.06(0.59-1.91) |
| 4 | 0.92(0.55-1.51) | 0.74(0.45-1.21) | 0.73(0.45-1.20) |
| 5+ | 1.60(1.03-2.47) | 1.08(0.68-1.71) | 1.07(0.67-1.70) |
| Parity | Hysterectomy before age 40 (n=1,653) | ||
| 0 | 0.36(0.28-0.48) | 0.42(0.32-0.55) | 0.41(0.31-0.54) |
| 1 | 0.81(0.66-0.99) | 0.80(0.65-0.98) | 0.81(0.66-0.99) |
| 2 | 1 | 1 | 1 |
| 3 | 1.10(0.89-1.35) | 1.03(0.84-1.28) | 1.04(0.84-1.29) |
| 4 | 1.19(0.92-1.53) | 1.01(0.79-1.31) | 1.03(0.80-1.32) |
| 5+ | 0.98(0.77-1.25) | 0.75(0.59-1.02) | 0.77(0.60-1.03) |
| Parity | Bilateral oophorectomy before age 40 (n=624) | ||
| 0 | 0.51(0.34-0.77) | 0.59(0.39-0.88) | 0.58(0.39-0.88) |
| 1 | 0.96(0.69-1.34) | 0.96(0.69-1.34) | 0.97(0.70-1.37) |
| 2 | 1 | 1 | 1 |
| 3 | 1.36(1.03-1.78) | 1.28(0.97-1.69) | 1.29(0.97-1.71) |
| 4 | 0.90(0.62-1.29) | 0.77(0.53-1.11) | 0.78(0.54-1.13) |
| 5+ | 0.86(0.59-1.26) | 0.68(0.46-1.00) | 0.70(0.47-1.03) |
Table 2: Unadjusted and adjusted odds ratios for the association of parity with premature menopause, hysterectomy, and bilateral oophorectomy before age 40 among 13,108 women in the NHANES 1999-2018.
Among 11,690 parous women, there was no significant association between parity and premature menopause and bilateral oophorectomy before age 40 after controlling for confounders listed above and adjusting for age at first and last childbirth [Tables 3 and 5]. But the OR (95% CI) for hysterectomy with a parity of 1 was 0.79 (0.63-0.99) versus 2 [Table 4].
| Exposure variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Parity | |||
| 1 | 1.63(1.03-2.56) | 1.58(0.98-2.57) | 1.64(0.94-2.86) |
| 2 | 1 | 1 | 1 |
| 3 | 1.17(0.66-2.08) | 1.08(0.60-1.96) | 1.10(0.58-2.08) |
| 4 | 0.92(0.55-1.51) | 0.74(0.45-1.22) | 0.77(0.45-1.35) |
| 5+ | 1.60(1.03-2.47) | 1.08(0.67-1.76) | 1.17(0.60-2.28) |
| Age at first childbirth (years) | |||
| <20 | 1.38(0.78-2.47) | 0.88(0.50-1.54) | 0.88(0.44-1.75) |
| 20-24 | 1.40(0.83-2.36) | 1.13(0.67-1.89) | 0.93(0.50-1.74) |
| 25-29 | 1 | 1 | 1 |
| 30+ | 0.95(0.43-2.08) | 1.06(0.48-2.34) | 1.09(0.47-2.52) |
| Age at last childbirth (years) | |||
| <25 | 0.83(0.51-1.35) | 0.71(0.44-1.15) | 0.67(0.41-1.08) |
| 25-29 | 1 | 1 | 1 |
| 30-34 | 0.52(0.33-0.82) | 0.54(0.34-0.86) | 0.54(0.33-0.88) |
| 35+ | 0.75(0.50-1.14) | 0.78(0.50-1.21) | 0.77(0.44-1.34) |
Table 3: Unadjusted and adjusted odds ratios for the association of parity, age at first childbirth, and age at last childbirth with premature menopause among 11,690 parous women in the NHANES 1999-2018.
| Exposure variables | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Parity | ||||
| 1 | 0.81(0.66-0.99) | 0.80(0.66-0.99) | 0.79(0.63-0.99) | |
| 2 | 1 | 1 | 1 | |
| 3 | 1.10(0.89-1.35) | 1.04(0.84-1.28) | 1.07(0.85-1.33) | |
| 4 | 1.19(0.92-1.53) | 1.03(0.80-1.32) | 1.18(0.90-1.55) | |
| 5+ | 0.98(0.77-1.25) | 0.77(0.60-0.98) | 1.11(0.83-1.48) | |
| Age at first childbirth (years) | ||||
| <20 | 3.45(2.70-4.43) | 3.16(2.41-4.15) | 2.08(1.52-2.85) | |
| 20-24 | 1.84(1.46-2.31) | 1.75(1.39-2.21) | 1.40(1.06-1.86) | |
| 25-29 | 1 | 1 | 1 | |
| 30+ | 0.23(0.12-0.44) | 0.23(0.12-0.46) | 0.46(0.23-0.91) | |
| Age at last childbirth (years) | ||||
| <25 | 1.39(1.19-1.63) | 1.27(1.08-1.49) | 1.16(0.98-1.38) | |
| 25-29 | 1 | 1 | 1 | |
| 30-34 | 0.53(0.44-0.63) | 0.54(0.45-0.65) | 0.62(0.51-0.74) | |
| 35+ | 0.16(0.11-0.22) | 0.16(0.11-0.22) | 0.19(0.14-0.27) | |
Table 4: Unadjusted and adjusted odds ratios for the association of parity, age at first childbirth, and age at last childbirth with experiencing hysterectomy before the age of 40 among 11,690 parous women in the NHANES 1999-2018.
| Exposure variables | Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|---|
| Parity | |||||
| 1 | 0.96(0.69-1.34) | 0.97(0.70-1.35) | 0.96(0.67-1.38) | ||
| 2 | 1 | 1 | 1 | ||
| 3 | 1.36(1.03-1.78) | 1.28(0.97-1.70) | 1.28(0.93-1.75) | ||
| 4 | 0.90(0.62-1.29) | 0.78(0.54-1.12) | 0.83(0.55-1.24) | ||
| 5+ | 0.86(0.59-1.26) | 0.69(0.47-1.02) | 0.87(0.56-1.36) | ||
| Age at first childbirth (years) | |||||
| <20 | 3.73(2.44-5.68) | 3.44(2.22-5.34) | 2.53(1.51-4.24) | ||
| 20-24 | 2.14(1.43-3.21) | 2.04(1.35-3.07) | 1.66(1.04-2.63) | ||
| 25-29 | 1 | 1 | 1 | ||
| 30+ | 0.41(0.16-1.07) | 0.44(0.17-1.13) | 0.70(0.26-1.84) | ||
| Age at last childbirth (years) | |||||
| <25 | 1.49(1.11-1.99) | 1.36(1.02-1.81) | 1.16(0.83-1.61) | ||
| 25-29 | 1 | 1 | 1 | ||
| 30-34 | 0.53(0.39-0.72) | 0.55(0.40-0.75) | 0.65(0.48-0.90) | ||
| 35+ | 0.25(0.16-0.40) | 0.26(0.17-0.41) | 0.35(0.22-0.55) | ||
Table 5: Unadjusted and adjusted odds ratios for the association of parity, age at first childbirth, and age at last childbirth with experiencing bilateral oophorectomy before the age of 40 among 11,690 parous women in the NHANES 1999-2018.
Figure 1 illustrates the adjusted association between continuous parity and premature menopause, hysterectomy, or bilateral oophorectomy before age 40. The overall nonlinear association of parity with hysterectomy or bilateral oophorectomy before age 40 was significant among nulliparous and parous women [Figure 1- (B) and (C)], while the adjusted association between parity and premature menopause was insignificant [Figure 1-(A) and (D)]. In addition, among parous women, continuous parity was nonlinearly associated with experiencing bilateral oophorectomy but not hysterectomy before the age of 40 [Figure 1- (E) and (F)].
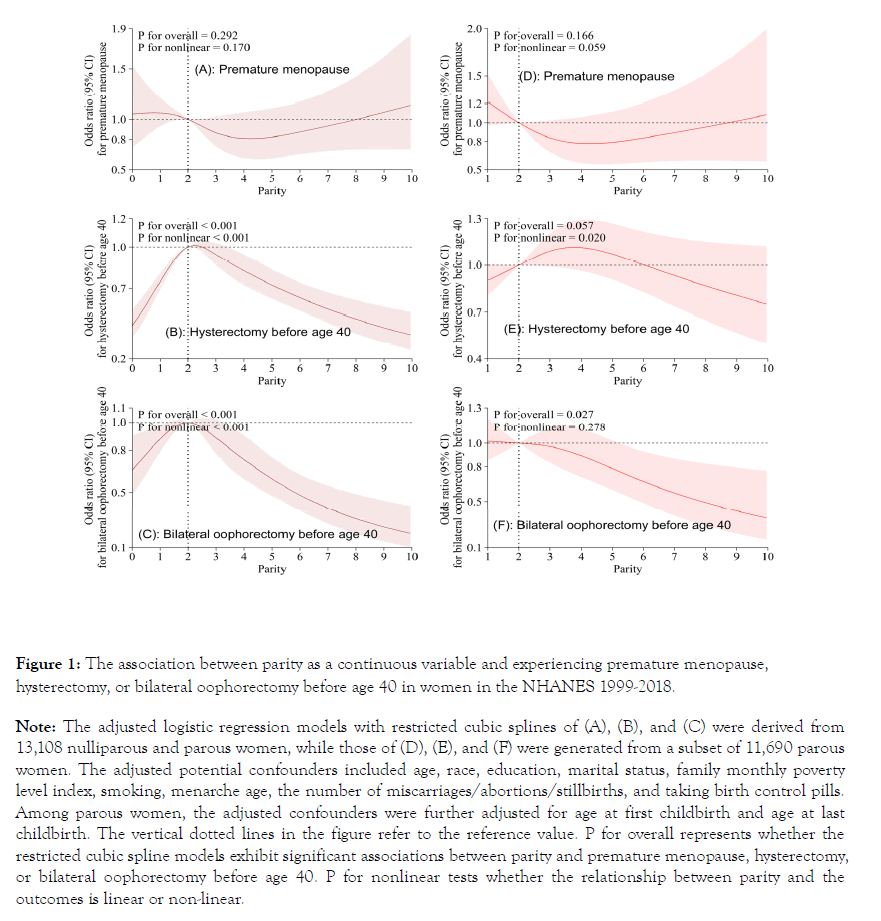
Figure 1. The association between parity as a continuous variable and experiencing premature menopause, hysterectomy, or bilateral oophorectomy before age 40 in women in the NHANES 1999-2018. Note: The adjusted logistic regression models with restricted cubic splines of (A), (B), and (C) were derived from 13,108 nulliparous and parous women, while those of (D), (E), and (F) were generated from a subset of 11,690 parous women. The adjusted potential confounders included age, race, education, marital status, family monthly poverty level index, smoking, menarche age, the number of miscarriages/abortions/stillbirths, and taking birth control pills. Among parous women, the adjusted confounders were further adjusted for age at first childbirth and age at last childbirth. The vertical dotted lines in the figure refer to the reference value. P for overall represents whether the restricted cubic spline models exhibit significant associations between parity and premature menopause, hysterectomy, or bilateral oophorectomy before age 40. P for nonlinear tests whether the relationship between parity and the outcomes is linear or non-linear.
Association between age at first childbirth and premature menopause, hysterectomy, or bilateral oophorectomy before age 40
A significant association was not found between age at first delivery and premature menopause, while negative associations were observed between age at first childbirth and hysterectomy or bilateral oophorectomy before age 40. Compared to those with age at first childbirth of 25-29 years, the adjusted ORs (95% CI) for hysterectomy were 2.08 (1.52-2.85), 1.40 (1.06-1.86), and 0.46 (0.23-0.91) for women with age at first childbirth of <20, 20-24, and 30+ years, and for bilateral oophorectomy, they were 2.53 (1.51-4.24), 1.66 (1.04-0.63), and 0.70 (0.26-1.84), respectively [Tables 3-5].
Figure 2-(B) and (C) presents the significant overall association between continuous age at first childbirth and hysterectomy or bilateral oophorectomy prior to age 40, without significant nonlinearity. However, the association between continuous age at first childbirth and premature menopause was insignificant [Figure 2- (A)].
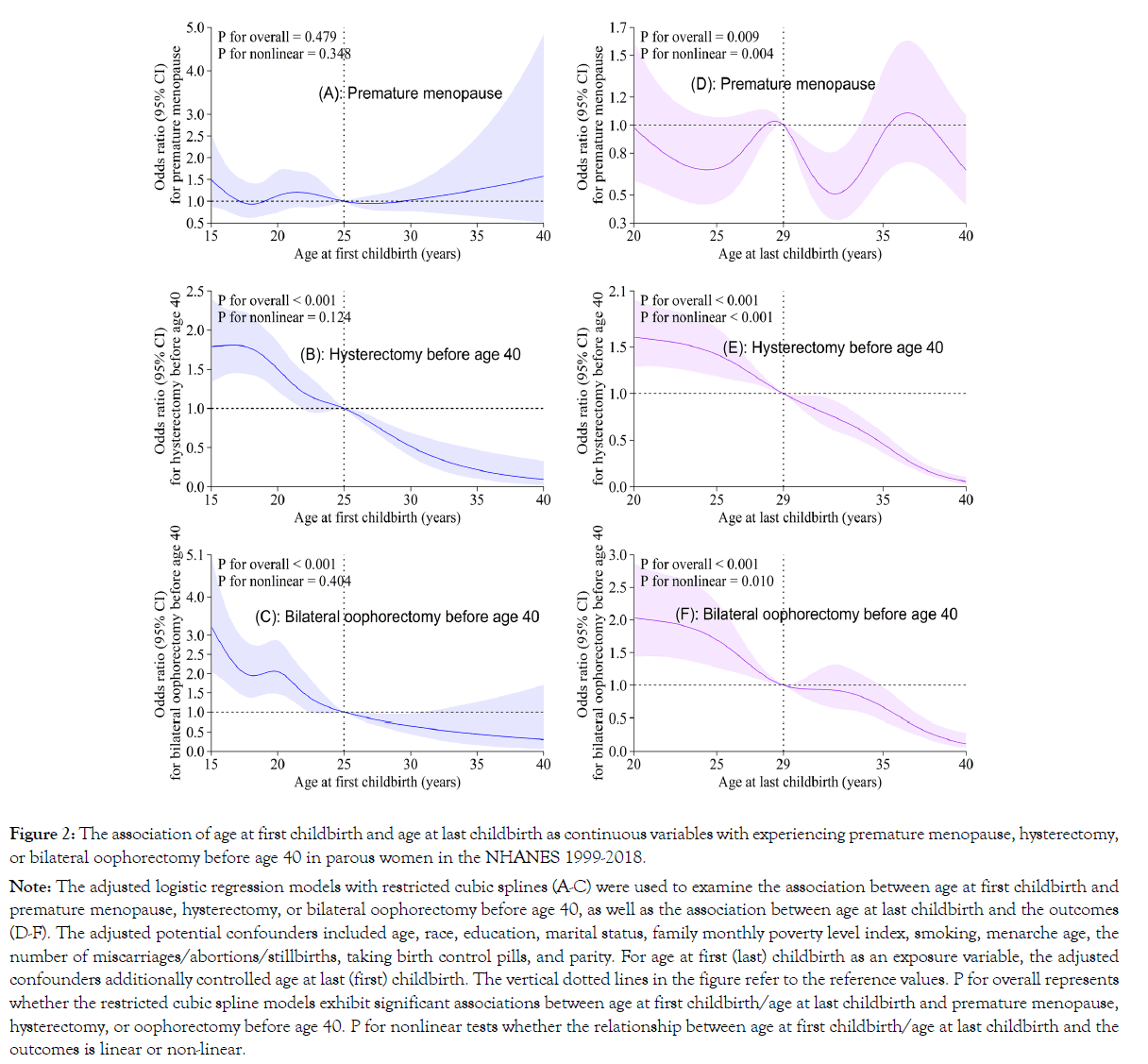
Figure 2. The association of age at first childbirth and age at last childbirth as continuous variables with experiencing premature menopause, hysterectomy, or bilateral oophorectomy before age 40 in parous women in the NHANES 1999-2018.
Note: The adjusted logistic regression models with restricted cubic splines (A-C) were used to examine the association between age at first childbirth and
premature menopause, hysterectomy, or bilateral oophorectomy before age 40, as well as the association between age at last childbirth and the outcomes
(D-F). The adjusted potential confounders included age, race, education, marital status, family monthly poverty level index, smoking, menarche age, the
number of miscarriages/abortions/stillbirths, taking birth control pills, and parity. For age at first (last) childbirth as an exposure variable, the adjusted
confounders additionally controlled age at last (first) childbirth. The vertical dotted lines in the figure refer to the reference values. P for overall represents
whether the restricted cubic spline models exhibit significant associations between age at first childbirth/age at last childbirth and premature menopause,
hysterectomy, or oophorectomy before age 40. P for nonlinear tests whether the relationship between age at first childbirth/age at last childbirth and the
outcomes is linear or non-linear.
Association between age at last childbirth and premature menopause, hysterectomy, or bilateral oophorectomy before age 40
Figure 2-(D) -(F) indicates the significant overall nonlinear inverse association between continuous age at last childbirth and premature menopause, hysterectomy, or bilateral oophorectomy prior to age 40. Women with age at last childbearing of 30-34 and 35+ years had adjusted ORs (95% CI) for hysterectomy of 0.62 (0.51-0.74) and 0.19 (0.14-0.27), respectively, compared to those having the last delivery at the ages of 25-29. The corresponding adjusted ORs (95% CI) for bilateral oophorectomy were 0.65 (0.48-0.90) and 0.35 (0.22-0.55), respectively [Tables 3-5].
Subgroup Analysis
According to the statistical criteria of P<0.05, significant interactions were only observed between parity by race and birth control usage among the interaction terms. The association between parity and hysterectomy before age 40 varied significantly across different races. For instance, nulliparity was associated with lower risks of hysterectomy in White, Black, and Hispanic women but not in other racial women. In addition, the association between nulliparity and hysterectomy before age 40 appeared more pronounced in women with a history of birth control pill use than those without [Figure 3].
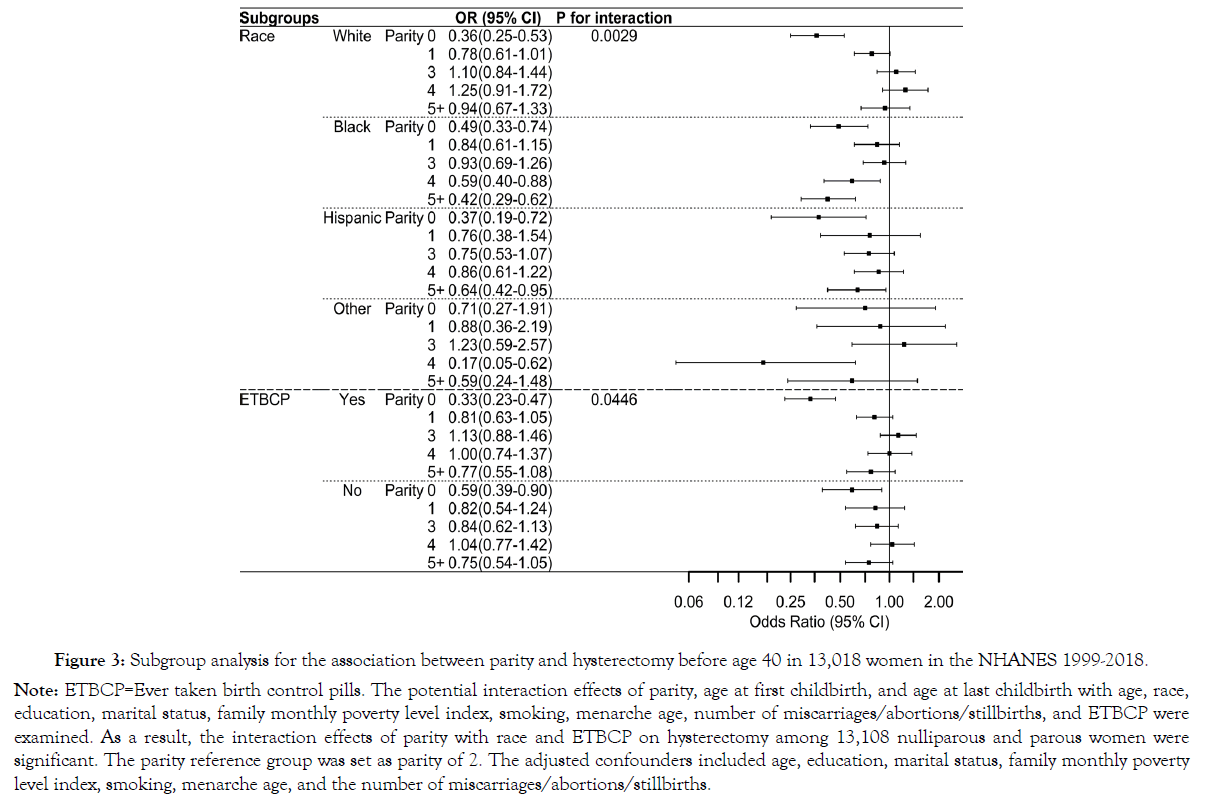
Figure 3. Subgroup analysis for the association between parity and hysterectomy before age 40 in 13,018 women in the NHANES 1999-2018.
Note: ETBCP=Ever taken birth control pills. The potential interaction effects of parity, age at first childbirth, and age at last childbirth with age, race,
education, marital status, family monthly poverty level index, smoking, menarche age, number of miscarriages/abortions/stillbirths, and ETBCP were
examined. As a result, the interaction effects of parity with race and ETBCP on hysterectomy among 13,108 nulliparous and parous women were
significant. The parity reference group was set as parity of 2. The adjusted confounders included age, education, marital status, family monthly poverty
level index, smoking, menarche age, and the number of miscarriages/abortions/stillbirths.
Sensitivity Analysis
Modifying exclusion criteria in sensitivity analyses produced similar ORs to those in the present study, without changing the overall interpretation [Supplemental Tables 1 and 2]. Besides, 12,876 participants had complete observations prior to covariate imputations. The sensitivity analysis findings based on these complete observations were consistent with the present findings [Supplemental Tables 3 and 4].
We found that nulliparity was associated with decreased risks of experiencing hysterectomy or bilateral oophorectomy before age 40 compared to a parity of 2, but no significant association was observed between nulliparity and premature menopause. In parous women, a parity of 1 was related to reduce risks of hysterectomy than parity of 2, but not with premature menopause or bilateral oophorectomy. Additionally, age at first childbirth was negatively associated with the risk of hysterectomy or bilateral oophorectomy, while age at last childbirth exhibited nonlinear inverse associations with the risks of premature menopause, hysterectomy, or bilateral oophorectomy before the age of 40.
Compared to a parity of 2, nulliparous women had lower risks of hysterectomy or bilateral oophorectomy prior to age 40; however, no significant association was observed between nulliparity and natural premature menopause in the present study. This finding is inconsistent with some studies [14,19,24]. A pooled analysis of over 50,000 postmenopausal women from nine observational studies conducted in the UK, Scandinavia, Australia, and Japan reported that nulliparity was significantly associated with higher risks of natural premature menopause compared to parity of 2+ (RR: 2.26, 95% CI: 1.84–2.77) [19]. The discrepancies in the findings may stem from variations in the reference groups (our study focused on parity of 2, while the study examined parity of 2+), participants' age, and race. However, Cooper et al.'s findings were in line with our research results, which showed hazard ratios of 1.89 (1.20-3.00), 1.57 (1.03-2.40), and 2.79 (1.80-4.34) for hysterectomy in women with parity of 1, 2, and 3+ respectively compared to nulliparity, although the outcome did not solely pertain to hysterectomy before the age of 40 [27].
We observed no significant association between parity and premature menopause in parous women. Although limited studies explored the relationship between parity and premature menopause, our findings are consistent with multiple previous studies investigating the association of parity with age at menopause and hysterectomy [14,15, 17, 23]. The Study of Women's Health Across the Nation, which included 3,302 women with a median age of 46 years, reported that the median age at natural menopause did not significantly differ among women with parity of 1, 2, 3, and 4+ (52.5, 52.5, 52.8 and 52.3 years respectively; P=0.688) [17]. A meta-analysis revealed that the hazard ratio for the association between each additional childbirth and hysterectomy was 1.10 (95% CI: 0.86-1.41) based on four cohort studies, while the OR for this association was 1.04 (95% CI: 0.94-1.14) based on four cross-sectional surveys [23]. However, this was inconsistent with our findings that giving one birth was related to a 21% decreased risk of experiencing a hysterectomy before the age of 40 in contrast to childbirth of two children.
Compared to women having their first childbirth at age 25-29, those who gave birth before age 20 or between 20 and 24 years had 2.1- and 1.4-fold elevated risks of hysterectomy and 2.5- and 1.7-times increased risks of bilateral oophorectomy prior to age 40. In contrast, a cross-sectional study of 3,328 Chinese women aged 25-69 years reported no significant association between age at first childbirth and hysterectomy, with ORs (95% CI) of 0.68 (0.43-1.07) and 0.75 (0.46-1.23) for age at first childbirth of 23-24 years and ≥24 years, respectively, compared to age at first childbirth ≤22 years [38].
Our findings show that age at first childbirth was not associated with natural premature menopause. Similarly, the results of two population-based studies in Britain, involving over 17,000 women, also indicated no significant association between age at first delivery (<20 and >34 years) and early natural menopause compared to age at first childbirth of 20-34 years [39].
Several studies reported that advanced maternal age was associated with delayed onset of menopause [40-42]. A cross-sectional survey of 948 postmenopausal women in Iran reported that younger age at last childbirth was related to younger age at menopause, with <25, 25-29, 30-34, and ≥35 years age at last childbirth being related to 46.0, 47.6, 47.9, and 48.9 years mean age at menopause, respectively (P=0.001) [40]. We unanimously demonstrated a nonlinear inverse relationship between age at the last childbirth and natural premature menopause, hysterectomy, or bilateral oophorectomy before age 40. Conversely, a Korean study found that women who gave birth for the last time at ages <25, 25-29, 30- 34, and ≥35 years had corresponding mean ages at menopause of 50.5, 50.7, 50.3, and 49.2 years (P=0.03), indicating that advanced maternal age was associated with earlier onset of menopause [43].
The precise biological mechanisms underlying the association between parity and hysterectomy, or oophorectomy, remain poorly understood [44, 45]. However, there are several plausible explanations. One hypothesis is that the association may be attributed to conditions such as uterine fibroids and endometriosis [46]. These disorders are also prevalent in women who have undergone childbirth and may necessitate hysterectomy as a potential treatment [47, 48]. In addition, women who have given birth may be at increased risk of experiencing heavy or prolonged menstrual bleeding, which can lead to various uterine disorders that necessitate surgery [49].
Regarding the mechanism underlying the association between young age at childbirth and hysterectomy, or oophorectomy, some scholars have suggested that early initiation of sexual activity may increase susceptibility to sexually transmitted infections, which can lead to pelvic inflammatory disease and other conditions that heighten risks of hysterectomy or oophorectomy [50-52]. Additionally, changes in hormone levels and ovarian function were assumed to be the cause of the link between age at last childbirth and premature menopause [53, 54]. The hormonal fluctuations that transpire during pregnancy and lactation may confer a safeguarding effect on a woman's oocytes, thereby postponing the onset of menopause [55].
The strengths of this study lay in its representative sample of women in the United States and its large sample size, spanning ten survey cycles. Additionally, the potential association of parity, age at first childbirth, and age at last childbirth with the risk of natural premature menopause, hysterectomy, and oophorectomy before age 40 has not been thoroughly investigated among American women despite previous studies examining the association of childbirth with age at menopause or hysterectomy. This present study addresses the existing research gap. Furthermore, we conducted comprehensive analyses of the associations, utilizing parity, age at first childbirth, and age at last childbirth as both categorical and continuous variables, as well as examining potential interactive effects and performing sensitivity analyses.
Regarding the limitations of this study, it should be noted that the exposure variables, the outcome, and the covariates were all selfreported through questionnaires. Consequently, non-differential measurement errors due to recall bias may have been unavoidable. In addition, not all survey cycles between 1999 and 2018 included data on covariates such as infertility, vaginal and cesarean deliveries, and breastfeeding among parous women. Therefore, these potential confounding factors were not incorporated into our models. Besides, due to the cross-sectional nature of NHANES, it cannot be confirmed that exposure occurred before the outcome. To address this temporal limitation, we excluded nulliparous women who underwent menopause before 32 (the 95th percentile for age at first childbirth in our study). Also, we applied the exclusion criteria of before 25 and 29 (the 75th and 90th percentiles, respectively) for age at first childbirth to ensure robust results.
Our findings suggest that nulliparity was associated with lower risks of hysterectomy or bilateral oophorectomy before age 40, but not natural premature menopause. Among parous women, early age at first childbirth, mainly before 20 years old, significantly increased the risk of hysterectomy or bilateral oophorectomy before reaching the age of 40. We also observed a solid nonlinear inverse association between age at last childbirth and premature menopause, hysterectomy, or bilateral oophorectomy prior to age 40. Overall, our findings provide some evidence for a broader comprehension of the persistent adverse effects of parity levels and young age at childbirth on women's health during their later years. Further investigation through additional cohort studies is necessary to validate the results obtained in our study.
The study did not receive any financial support.
No interest in conflict existed.
Zailing Xing was responsible for the study's conception and design, data analysis, and the initial draft of the manuscript. Dr. Russell S. Kirby contributed significantly to the conceptualization, design, modification, and supervision of the paper. Both authors made contributions to the data interpretation, engaged in critical editing of the work to enhance its intellectual substance, and granted their permission for the final version that was submitted.
The data acquisition from the participants was approved by the Ethics Review Board of the National Center for Health Statistics of the Centers for Disease Control and Prevention (NHANES 1999- 2018, registration number: Protocol #98-12, #2005-06, #2011-17). Each participant signed a written form of informed consent.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Citation: Xing Z, Kirby RS (2023) Parity, Age at Childbirth, and Premature Menopause, Hysterectomy, and Bilateral Oophorectomy before Age 40 in Women in the United States: National Health and Nutrition Examination Survey 1999–2018. 12(12):702.
Received: 29-Nov-2023, Manuscript No. 28255; Editor assigned: 01-Dec-2023, Pre QC No. 28255; Reviewed: 17-Dec-2023, QC No. 28255; Revised: 22-Dec-2023, Manuscript No. 28255; Published: 28-Dec-2023 , DOI: 10.35248/2167-0420.23.12.702
Copyright: © 2023 Xing Z & Kirby RS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Sources of funding : The study did not receive any financial support.