Journal of Glycobiology
Open Access
ISSN: 2168-958X
ISSN: 2168-958X
Research Article - (2023)Volume 12, Issue 6
Published data on the properties and possible applications of the Lectin(s) from Trilepisium madagascariense seed is still limited. This study aimed to partially characterize and investigate the seed extract of T. madagascariense for antibacterial activity against Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli, using agar well diffusion method. The extract was partially purified by Sephadex G-75 filtration and characterized, to obtain a partially purified Trilepisium madagascariense Seed Lectin (ppTMSL).The ppTMSL was found to agglutinate all human erythrocytes (blood groups A, B, and O), showing no blood group specificity; although there was statistical increase (P<0.05) in its activity on blood group O erythrocytes while comparing the natural logarithm of the mean of specific activities of the TMSL with the other blood groups erythrocytes. ppTMSL was however found to betteragglutinate rabbit erythrocyte. Molecular weight estimation suggests a heterodimer of 22 kDa and 24 kDa respectively in the ppTMSL, having 7-8 and 25°C-50°C as the optimum pH and temperature for its maximum hemagglutinating activity. ppTMSL was found to lack sensitivity against all the bacterial isolate tested. The results will provide useful guidelines for further research on TMSL and its possible applications.
Lectin; Trilepisiummadagascariense; Partial purification; Characterization; Anti-bacterial activity
Lectins are non-immunogenic carbohydrate-recognizing proteins that bind to glycoproteins, glycolipids, or polysaccharides with high affinity and exhibit remarkable ability to agglutinate erythrocytes and other cells [1]. Lectins bind to carbohydrates reversibly and non-covalently without inducing any change in the bound carbohydrate. Most lectins are members of families with defined “Carbohydrate-Recognition Domains” (CRDs) that have evolved from shared ancestral genes, often retaining specific features of primary amino acid sequence or three-dimensional structure.
Lectins are found in virtually all parts of plant such as the bark, stem, seed, leaf and also root [2]. They are the first and most extensively studied lectins due to their broad distribution [3]. Numerous plant lectins have been purified and their bioactivities and sugar-binding specificities have been characterized in detail.
The content of lectin varies among different organisms and may differ among different cultivars of the same plant species. The content is higher in some parts of the plants than others [4]; for example, the lectin yields were 0.75 and 3.9 g/kg in Astragalus mongholicus roots [5], and Remusatia vivipara tubers [6], respectively.
Some lectins have been reported to possess antibacterial potentials [7-9], for example described that lectins are able to promote growth inhibition and death of human pathogenic bacteria as well as interfere with their adherence and invasive capacities by binding to glycoconjugates present at bacterial or host cell surfaces, preventing the interaction between microorganism and human cells.
The interaction of lectins with molecules from bacteria cell wall, such as teichoic acids, peptidoglycan, and lipo-polysaccharide, may cause an inhibitory effect on their growth [10]. Also, lectins can affect cell permeability by damaging membrane integrity, causing the death of the microorganism [11].
Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli are among the common disease-causing bacteria that require the development of new drug due to their range of resistance against common antibiotics [12]. S. aureus and B. cereusare are gram-positive bacteria. S. aureus that causes skin and soft tissue infections such as abscesses (boils), furuncles, and cellulitis, and more serious infections like bloodstream infections, pneumonia, or bone and joint infections [13]. B. cereus is a food-borne pathogen that causes gastrointestinal illness via toxin production [14]. E. coli, and P. aeruginosa, among the gram-negative bacteria also have huge clinical significance. E.coli, cause intestinal or extra-intestinal infections, including severe invasive disease such as bacteremia and sepsis [15], whereas P. aeruginosa is a frequent cause of nosocomial infections such as pneumonia, Urinary Tract Infections (UTIs), and bacteremia [16].
Because of available antibacterial failure to treat infectious diseases, many researchers have focused on the investigation of natural products as source of new bioactive molecules. Plant lectins have applications in pharmaceutical research such as cell growth and regulation, Cell induction, fertilization and agglutination of cells and bacteria and immune recognition process because of their specificity to different sugars. They are therefore considered strong candidates for therapeutic use [17].
This study aimed to partially characterize and investigate seed extract of T. madagascariense for antibacterial activity against B. cereus, S.aureus, P. aeruginosa and E. coli.
Preparation of T.madagascariensecrude extract
Fresh ripened fruits of Trilepisium madagascariense were obtained from Ile-Ife, Nigeria and identified at the Herbarium laboratory, Department of Botany, Ahmadu Bello University, Nigeria. Their seeds air-dried at room temperature until their weights were constant. The dried seeds were then ground into powder which was defatted using petroleum ether. The resulting fine powder was then extensively extracted in Phosphate Buffered Saline (PBS), pH 7.2 on magnetic stirrer and centrifuged at 6,000×g for 15 minutes. The supernatant (containing crude extract of the seed) was collected into a sample bottle and stored at -20°C. It was then lyophilized for storage and subsequent work.Purification of lectin by gel filtration on Sephadex G-75
Swollen Sephadex G-75 resin was packed into a chromatographic column and equilibrated with PBS. The lyophilized extract was dissolved in PBS and then layered on the column. The elution was carried out using PBS. Twenty fractions, each of 3 ml were collected at 9 ml/hr (3 ml/20 minutes). The fractions were monitored for protein by measuring the absorbance at 280 nm, and hemagglutination assay. Fractions 4-10 were pooled for subsequent investigations, and called partially purified Trilepisium madagascariense seed lectin ppTMSL.
Total protein estimation by Lowry method
Protein concentration of both the crude extract and the ppTMSL were determined using the method of Lowry, using Bovine Serum Albumin (BSA) as standard. Briefly, the method involves mixing the sample with copper sulfate (CuSO4), followed by the addition of Folin-Ciocalteu reagent. This results in the formation of a blue color complex, the intensity of which is proportional to the protein concentration. BSA is used as a standard to create a calibration curve for quantifying the protein concentration in the lectin-containing extract based on the color change.
Characterization of T.madagascariense Activity assays: The lectin activity of T. madagascariense was established by haemagglutination and inhibition assays. Haemagglutination activity was assessed using glutaraldehyde- fixed erythrocyte suspensions according to the method of Sharon and Lis, [18]. Human blood group A, B and O were used, and rabbit blood also. A serial dilution of the sample was performed into 24 wells for each blood type. Each well was then incubated with 2% erythrocyte suspension for 2 hour to allow for agglutination.
Molecular weight determination: The molecular weight of the sample was estimated by SDS-PAGE (Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis) under denaturing conditions according to Laemmli [19]. After dissociation of the protein with 0.1% SDS, the gel was run on a Mini-Protein III electrophoretic system at a constant voltage (50 V) and protein bands were visualized by staining with 0.05% Coomassie Brilliant Blue R250. Molecular mass was estimated by comparing the electrophoretic mobility of the sample against Rainbow molecular mass marker proteins.
Effect of temperature and pH on the activity of the purified lectin: The effect of temperature changes on the lectin was assessed by incubating the purified lectin over a temperature range of 25-70°C with increments of 5°C in a water bath at pH 7.2. Samples were drawn after 30 minutes of incubation, cooled to room temperature and assayed for hemagglutination. The effect of pH on the activity of TMSL was determined using buffer ranging from pH 3-11 at room temperature, namely; glycine-HCl (pH 3), sodium acetate (pH 4 and 5), sodium phosphate (pH 6), PBS (pH 7), TrisHCl (pH 8), glycine-NaOH (pH 9) and carbonate-bicarbonate (pH 10 and 11). The lectin (100 μl) was incubated with 100 μl of the different buffer solutions for 30 minutes at room temperature and then assayed for agglutination with 2% erythrocytes suspension [20].
Standardization of bacteria isolate: Test bacteria were clinical isolates of Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli obtained from the microbiology laboratory, Ahmadu Bello University Teaching Hospital, Nigeria. They were sub-cultured and incubated at 37°C for 24 hours. The discrete colonies from the sub-cultured isolates were collected using sterile wire loop and introduced into 5 ml normal saline and standardized by comparing the turbidity of the bacterial suspension with 0.5 Mc Farland standards to obtain 1.5 × 108 cfu/ml bacteria culture.
Antibacterial susceptibility test of ppTMSL: Susceptibility test of the isolates was carried out using Agar well diffusion method [21]. Standardized inoculum (0.2 ml) was dispensed and spread on each media in petri dishes. Four wells were made in each plate (for each of the test organism) using sterile cork borer (6 mm diameter). Aliquots of the ppTMSL were dispensed into each well with percentage concentrations 12.5, 25, 50 and 100 respectively. The plates were allowed to stand for one hour to allow adequate diffusion of the extracts, and then incubated at 37°C for 24 hours. Antibiotic ciprofloxacin discs ware used as a positive control. Each test was done in triplicate. Antibacterial activity was determined by measuring the zones of growth inhibition (clear zone) surrounding the wells in millimeter using transparent meter rule.
Fractionation and molecular weight determination of ppTMSL
Fractions 4 to 10 were pooled together from the single-step fractionation protocol (Figure 1) that resulted in what we called ppTMSL. Whereas the crude extract had 2.071mg/mL protein, the ppTMSL had 0.207mg/mL. The purification protocol resulted in a purification fold of 40 and a yield as 35% (Table 1). The Electrophoregram of ppTMSL (Plate I), shows two bands corresponding to molecular weight of 22 kDa and 24 kDa as extrapolated from the plot of logarithm of molecular weight against retention factor of the molecular weight marker proteins.
| Sample | Volume (mL) | Protein concentration (mg/mL) | HA (U/mL) | Total HA (U) |
SHA(U/mg) | Purification fold | Yield (%) |
|---|---|---|---|---|---|---|---|
| Crude extract | 1000 | 2.071 | 64 | 1,28,000 | 30.9 | 1 | 100 |
| Gel filtration (Sephadex G-75) | 175 | 0.207 | 256 | 44,800 | 1236.7 | 40 | 35 |
Note: Hemagglutinating Activity (HA) the number of units of hemagglutinating activity was defined as the reciprocal of the highest dilution of sample that showed agglutination of blood group O erythrocytes; Specific Hemagglutinating Activity (SHA) is HA divided by the protein content, Purification fold is estimated by dividing a specific activity and the previous one yield is the is the % quotient of a total activity and that of the previous one multiplied.
Table 1: Purification table of TMSL.
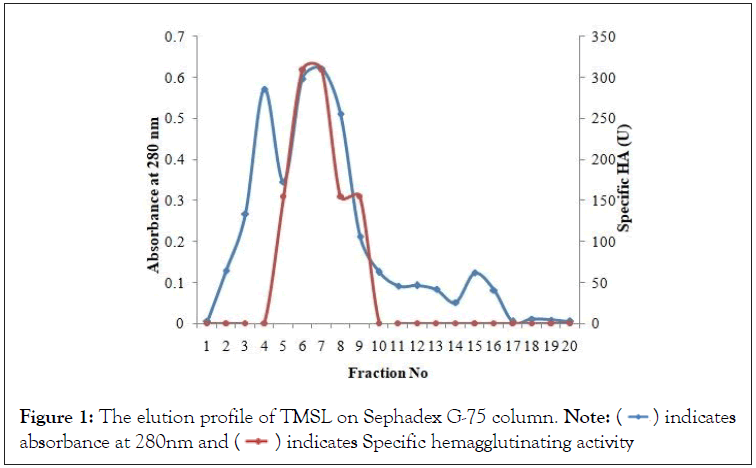
Figure 1: The elution profile of TMSL on Sephadex G-75 column. Note:  indicates
absorbance at 280nm and
indicates
absorbance at 280nm and  indicates Specific hemagglutinating activity
indicates Specific hemagglutinating activity
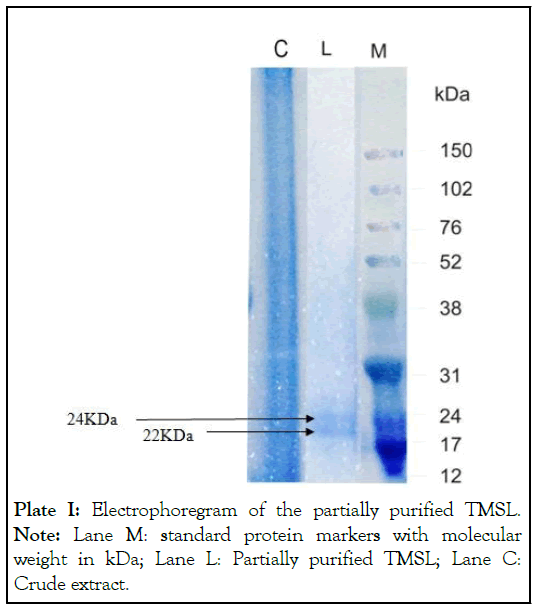
Plate I: Electrophoregram of the partially purified TMSL. Note: Lane M: standard protein markers with molecular weight in kDa; Lane L: Partially purified TMSL; Lane C: Crude extract.
Hemmaglutination assayLectin activity of the pooled fractions was establishes by agglutination assays using fixed erythrocytes of human (blood group A, B and O) and rabbit. The sugars on the surface of erythrocytes interact with ppTMSL resulting in agglutination. This is evident by the formation of a carpet layer on the bottom of the wells of a microtiter plate. On the other hand, in the absence of sufficient concentration of lectin to produce agglutination was established by a distinctive red button on the bottom of the well. The reciprocal of dilution was calculated as titer value, which reflects lectin activity. The higher the titer value, the higher the lectin activity.
The ppTMSL agglutinated all the blood groups tested (Table 2), hence, it’s non-blood group specific. On the consideration of the natural logarithm of their specific activity, using Bonferroni test to compare the difference among the mean of the different blood agglutination, those of human blood group A and B were not statistically different from each other, but they were lower than that of human blood group O, whereas the natural logarithm of their specific activity with respect to rabbit blood is statistically higher than those of all the human blood groups.
| Blood group | HA(U/mL) | Protein Conc.(mg/ml) | SHA (U/mg) | ln SHA |
|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 |
| Human blood group A | 64 | 0.207 | 309.179 | 5.734a |
| Human blood group B | 64 | 0.207 | 309.179 | 5.734a |
| Human blood group O | 256 | 0.207 | 1236.715 | 7.120b |
| Rabbit blood | 4096 | 0.207 | 19787.44 | 9.893c |
Note: Protein concentration was determine using Lowry assay, (SHA) Specific Hemagglutinating Activity is HA divided by the protein concentration.
ln SHA, natural logarithm of SHA was:
a In Specific activity observed with human blood group A and B is statistically lower than those of human blood group O and rabbit blood (P<0.05 were considered as significant); b In Specific activity observed with human blood group O is statistically lower than that of rabbit blood (P< 0.05 were considered as significant); c In Specific activity observed with rabbit blood is statistically higher than that of all the human blood groups (P<0.05 were considered as significant).
Table 2: The Blood Group Specificity of TMSL
Effect of temperature ph on the hemagglutination activity of TMSL
The ppTMSL has maximum hemagglutination activity between 25°C and 50°C (Figure 2A) and between pH 7 and 8 (Figure 2B). Absolute hemagglutination activity, corresponding to 256 HU with blood group 0 erythrocyte. Up to half of the activity was lost beyond 50°C and the lectin was found to loose its hemagglutination activity totally at 70°C. Agglutination activity was also lost below pH 4 and above pH 11.
Figure 2: A shows the effect of temperature variation on the hemagglutination activity of ppTMSL; B shows the effect of pH variation on the hemagglutination activity of ppTMSL .
The antibacterial test of TMSL
The ppTMSL was found to lack toxicity against all the four bacteria tested (Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli) (Plates II and III). In all the cases, the control experiment shows noticeable inhibition zones.
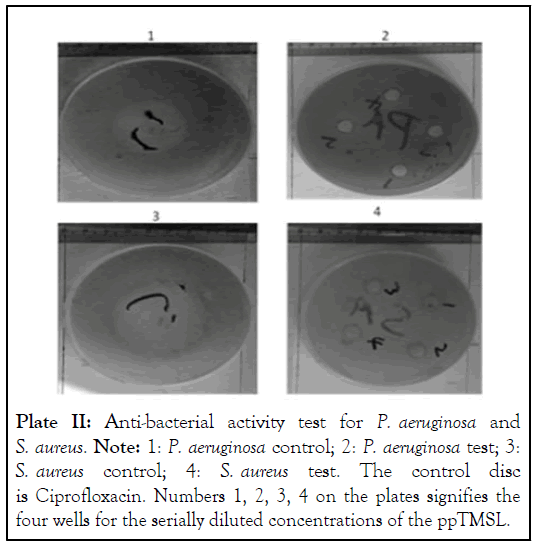
Plate II: Anti-bacterial activity test for P. aeruginosa and S. aureus. Note: 1: P. aeruginosa control; 2: P. aeruginosa test; 3: S. aureus control; 4: S. aureus test. The control disc is Ciprofloxacin. Numbers 1, 2, 3, 4 on the plates signifies the four wells for the serially diluted concentrations of the ppTMSL.
Plate III: Anti-bacterial activity test for B. cereus and E. coli. Note: 1: B. cereus control; 2: B. cereus test, 3: E. coli control; 4: E. coli test. The control disc is Ciprofloxacin. Numbers 1,2,3,4 on the plates signifies the four wells for the serially diluted concentrations of the ppTMSL.
Lectins are a subject with immense potential and of intense investigations. As more lectins are isolated and further studies are conducted on the biological activities and mechanisms of action of lectins, the production of lectins can be improved and new applications of lectins can be found and explored for significant contributions in various fields of biology.
Blood group sensitivity of lectins is influenced by any modification or substitution on the carbohydrate binding site of the lectin. This affects the number of contacts with carbohydrates and depth of the sugar binding sites, hence influencing binding specificity [1]. The observed differences in ppTMSL activity with different blood groups may be attributed to differences in carbohydrate-lectin binding interactions which can be due to differences in nature and order of carbohydrates presented on the erythrocyte surfaces of the different blood type [22]. The hemagglutinating potential of ppTMSL is similar to that of Aplysina lactuca [23], Quercus fusiformis [1], Bryopsis plumose [24], Kalanochoe crenata [25] and other plant lectins that are also non-blood group specific. The ppTMSL is therefore non-blood group specific, though it has higher affinity for rabbit erythrocytes, among the examined blood types. Human red blood cells may contain carbohydrate components on the cellular surface binding sites that are relatively less recognized by the ppTMSL binding site. On the other hand, the carbohydrates found on the cellular surface of rabbit red blood cells may contain carbohydrate units in a structure and position more specific and with higher affinity for the binding of ppTMSL, subsequently increasing rabbit erythrocyte agglutination.
Lectins commonly elicit ranging activities under different pH conditions because of the protonation and deprotonation of their side chains amino acids and their resulting reactivities [26]. Adenike and Eretan [25] reported the optimum pH for maximum activity of Kalanochoe crenata leaf lectin to be between 2 to 7.5. Dresch [27] isolated two lectins from Axinella corrugata (ACL-I and ACL-II) which was subjected to pH variation ranging from pH 1.0 to 11.0. They were found to maintain maximum activity, between pH 2.0 and 6.0. Generally, literature reports shows varying effect of pH on the activity of plant lectins. Morus rubra [26] and Ipomoea asarifolia [28] leaf lectin crude extracts were reported to have optimum activity at pH 7.5. The effect of pH on lectin activity of ppTMSL was quite similar to that of Chorchorus olitours lectin that exhibited an optimum agglutination activity from pH 7.2 to 8.0 and Aplysina lactuca lectin [23]. The ppTMSL lost its activity after heating for 30 mins beyond 70°C which is fairly close to A. lactuca lectin that have optimum activity up to 60°C, after which the activity decreases progressively. Apuleia leiocarpa seed lectin [8] and the coagulant lectin from Moringa oleifera seeds [29] that showed fairly constant even when being heated up to 100°C. The compromise in ppTMSL hemagglutinating activity with respected to pH and temperature variation may be due to disruption of the non-covalent interactions responsible for the stability of its tertiary structures.
Several lectins exhibit antibacterial activity, though not always against all kinds of bacteria species. Some studies demonstrated that components of the bacterial cell wall, such as Lipopolysaccharides (LPS), peptidoglycans and teichuronic and teichoic acids, are target sites for the antibacterial effects of lectin [30].
Quadri [31], reported that purified Indigofera heterantha lectin lack significant antibacterial activity on four (Pseudomonas spp. Shigella bodyi, Streptococci,and Salmonella typhi) out of the eight strains of human pathogenic bacteria examined. Pompeu et al. [32], also investigated the antibacterial activity of Chenopodium quinoa seed lectin, and antibacterial activities in only on three out of eleven bacterial strains. ppTMSL may therefore LPS composition, one or more of the sugars for which the lectin showed specificity. Aspergillus gorakhpurensis in the experiment of Singh [33], lacks sensitivity against P. aeruginosa, although other tested strains namely: B. cereus, S. aureus and E. coli presented zones of inhibition.
For ppTMSL, since observable antibacterial activity was not recorded in all the isolates tested, it could be imply that the investigated bacteria isolates do not have, in their cell wall, the sugars for which the lectin showed specificity or that the carbohydrates are not assessable to ppTMSL. Also a more concentrated ppTMSL may be required to attain the minimum concentration needed for growth inhibition.
The ppTMSL was found to be non-blood group specific as it agglutinated all the tested blood groups. It was found to have optimum pH and temperature for maximum activity as 7-8 and 25-50°C respectively. SDS PAGE analysis reveals that ppTMSL is a dimer of to 22 kDa and 24 kDa. The ppTMSL was found to lack activity against clinical isolates of the two gram-positive bacteria (Bacillus cereus and Staphylococcus aureus) and two gram- negative bacteria (Pseudomonas aeruginosa and Escherichia coli) tested.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Fasogbon IV, Yakubu MN, Mustapha A, Obi C, Tanko N, Zenoh DA, et al. (2023) Partial Purification, Characterization and Investigation of the Anti-Bacterial Activity of Trilepisium madagascariense Seed Lectin. J Glycobiol. 12:267.
Received: 17-Oct-2023, Manuscript No. JGB-23-27618; Editor assigned: 20-Oct-2023, Pre QC No. JGB-23-27618 (PQ); Reviewed: 03-Nov-2023, QC No. JGB-23-27618; Revised: 10-Nov-2023, Manuscript No. JGB-23-27618 (R); Published: 17-Nov-2023 , DOI: 10.35841/2168-958X.23.12.267
Copyright: © 2023 Fasogbon IV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.