Medical & Surgical Urology
Open Access
ISSN: 2168-9857
ISSN: 2168-9857
Research Article - (2023)Volume 12, Issue 4
Objective: To present the epidemiological, diagnostic and therapeutic profile of patients with testicular cancer in hospitals in the Thies region.
Patients and methods: We conducted a descriptive study from January 1st, 2015 to December 31st, 2022. We described the clinical features and treatment of cases managed in different urology departments in the Thiès region.
Results: We collected data on 16 cases of testicular cancer in the study period. Mean patients age was 27 ± 9.5 years. The age group most affected was 20 to 39 years old. The circumstances of discovery were an enlarged scrotum (14 cases), Scrotal pain (1 patient) and 1 case of unilateral scrotum vacuity associated with inguinal swelling. Ultrasound was performed in all patients. The dosage of tumor markers (alpha-feto-protein, beta-HCG) was carried out in 15 patients. All our patients underwent inguinal orchiectomy. Pathology examination noted 9 cases of seminoma germ cell tumor’s and 4 case of embryonal carcinoma. Six patients had secondary lymph node and pulmonary localizations. Chemotherapy was performed in 7 patients and no patient received radiotherapy. With an average follow-up of 6 months, 5 patients died.
Conclusion: Testicular cancer is rare in thies region Senegal. Inguinal orchiectomy is the standard treatment. This cancer is radio-chemo-sensitive.
Testicular; Diagnostic; Treatment; Thies region
With 75,000 new cases worldwide, testicular cancers are placed 20th in terms of incidence of all cancers combined [1]. Their incidence worldwide varies between 0.2 and 9.2 cases/year per 100,000 men [1,2]. The exact incidence of Testicular cancers in Senegal is unknown [3]. In Africa, African publications were made and the incidence was eestimated to range from (0.3–0.6/100,000) [3-5]. Our objective was to present the epidemiological, diagnostic and therapeutic aspects of testicular cancer in the Urology-Andrology departments of hospitals in the Thies region, Senegal.
We carried out a descriptive study between January 2015 and December 2020. Data were collected from patient medical records, operating room and hospitalization registries. We included patients operated on and hospitalized for testicular cancer with a pathology confirmation using the surgical specimen. The variables studied were the year and age at the time of diagnosis, the personal and family history of the patients, the circumstances of discovery, the physical finding, the contributing factors, the results of additional examinations, the treatment and post-therapeutic evolution. The classification of our patients and the management were per the CCAFU 2017 recommendations. Data were entered and analyzed with Microsoft Office Excel 2007 software.
During the study period, 16 cases of testicular cancer were treated, representing an annual frequency of 2.6 cases/year. Mean patients age was 27 years ± 9.5. The most affected age group was 20 to 39 years old. The risk factors found were a history of cryptorchidism (12.5%) and orchitis (12.5%). The mean time to consultation was 6.3 months. The circumstances of discovery were unilateral enlarged scrotum in 14 patients (75%), unilateral testicular pain in 1 patient (6.25%) and an inguinal mass in 1 patient (6.25%). Physical examination revealed a testicular mass in 93.7% of patients and bilateral inguinal lymphadenopathy in one patient (6.25%). Ultrasound was performed in all patients and confirmed the testicular mass. The dosage of tumor markers (alpha-feto-protein, beta-HCG) was carried out in 15 patients (Table 1). Inguinal orchiectomy was performed in all patients and associated bilateral lymph node dissection in one patient. The main histological types found were seminmotous germ cell tumour (56.25%), embryonal carcinoma and mixed tumour (Figure 1). According to the AJJCC 2017 classification, 56.25% of tumors were localized, 25% of patients had lymph node involvement and 18.75% presented visceral metastases (Figure 2). Chemotherapy was performed in 25% of patients. No patient underwent radiotherapy. Mortality at 6-month follow-up occurred in 5 patients.
| Characteristics | Effective | Percentage |
|---|---|---|
| Risk factors | ||
| Cryptorchidism | 2 | 12.5% |
| Orchits | 2 | 12.5% |
| Clinical presentation | ||
| Scrotal mass unilateral | 12 | 75% |
| Scrotal mass bilateral | 1 | 6.25% |
| Scrotal gravity | 1 | 6.25% |
| Testicular pain | 1 | 6.25% |
| Inguinale mass and bursa vacuity | 1 | 6.25% |
| Testicular nodule | 1 | 6.25% |
| Serum tumour markers | ||
| Alpha-Fetoprotein (AFP) >> (normal<7UI/l) | 10 | 62.5% |
| Total Human Chorionic Gonadotropin (HCGt) >> (normal<7UI/l | 8 | 50% |
| Lactate Dehydrogenases (LDH) >> (normal=207-414UI/L) | 7 | 43.75% |
| Testicular ultrasound | 16 | 100% |
| CT scan | 15 | 93.75% |
| Inguinal orchidectomy | 16 | 100% |
| Right orchidectomy | 6 | 37.5 % |
| Left orchidectomy | 9 | 56.25 % |
| Bilateral orchidectomy+Retroperitoneal lymph node dissection | 1 | 6.25% |
| Treatment complementary | ||
| Chemiotherapy | 7 | 43.75% |
| Radiotherapy | 0 | 0% |
| Evolution (6 months) | ||
| Stable | 8 | 50% |
| Recurence and progression tumorale | 3 | 18.75% |
| Death | 5 | 31.25% |
Table 1: Distribution of patients according epidemiological, diagnosis and therapeutic aspects.
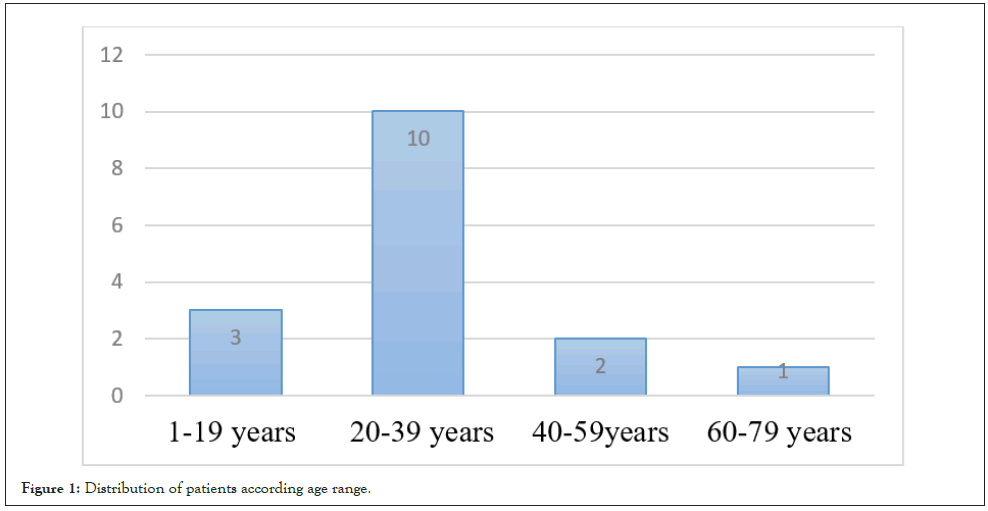
Figure 1: Distribution of patients according age range.
Figure 2: Mixed germ cell tumour aspect.
Epidemiology
The incidence of Testicular cancer varies from region. It was higher in northern Europe than in Asia and Africa [6]. Testicular cancer is rare in black people [3]. In the United States, testicular cancer is commonnest in white individuals (6.9 affected individuals per 100,000 males) than in African Americans (1.2 affected individuals per 100,000 males) [7]. We reported 16 cases in, giving an annual frequency of 2.6 cases per year. This rarity has been noted in Senegal as in Sub-Saharan Africa [3-5]. Kane, et al. [4] in Senegal as well as Ugwumba, et al. [8] in Nigeria reported respectively 17 cases in 15 years and 24 cases in 19 years. In France, the incidence is 4.5 cases per year/100,000 men [9]. Around 2,200 new cases of testicular cancer are diagnosed in France each year [9,10].
It is a cancer of young male, the age groups most affected were 21 to 40 years old [3-5,9]. This corroborates with most African series and in Europe where the peak incidence of this cancer is between the third and fourth decade [3-5,10,11]. The average age, in the series [11] in Cote d’Ivoire was 23.4 years and that of Kane, et al. [4] was 27 ± 9.5 years.
In our series, cryptorchidism was the only consensual risk factor found in 12.5% patients. According to some author, Cryptorchidism increases the risk of both ipsilateral and contralateral testicular cancer [12]. Other risk factors have been reported in the literature such as subfertility or infertility, testicular atrophy, family history of testicular cancer (1st degree) [9,13,14].
Diagnostic
Enlarged scrotum is the main mode of revelation of this affection in our series [15] reported that 79.5% of patients consulted for painless testicular swelling. Other modes of revelation have been reported in the literature such as testicular pain or abdominal mass with empty scrotum [3,5]. Most often, testicular cancer is suspected by palpation of a hard, asymptomatic scrotal mass, sometimes following a painful episode. There is no preferential laterality. It can be discovered during a scrotal ultrasound indicated for testicular trauma, subfertility or gynecomastia [2,9]. Testicular ultrasound coupled with physical examination helps to distinguish intra and extra testicular lesions [2,9]. More rarely, the diagnosis could be evoked in the case of respiratory distress or the appearance of a third lymph node [9]. The bilateral involvement reported in our series is exceptional.
Serum tumor markers such as total beta-human Chorionic Gonadotropin (hCGt), serum Alpha-Feto-Protein (AFP), and Lactate De--Hydrogenase (LDH) should be recommended before and after orchiectomy [9,16]. Alpha-Feto-Protein (AFP) was elevated in 10 patients (62.5%). In the series of Kane, et al. [4], Alpha-Feto-Protein (AFP) was elevated in only 5 patients. LDH was elevated in patients, indicating an advanced tumor. Enlarged inguinal orchiectomy is the standard treatment [2,9,16]. Pathology examination of the surgical specimen indicates the histological type according to the WHO 2016 classification [17,18]. Immunohistological analysis is recommended in case of doubt [9]. “Pure” seminoma germ cell tumors Tri-Glyceride’s (TG’s) are the most represented in our series (56.25%). Kane, et al. [4] found 88.4% non-seminoma tumors [19], reported cases of primary lymphomas of the urinary tract. Some authors reported the predominance of seminmotous tumors [5,11]. According to the American Joint Committee on Cancer (AJCC) 2017 classification [18,19] 56.25% of patients were classified as localized stages and 25% of patients had retroperitoneal lymph node involvement and visceral metastatis accounted for 18.75% of cases.
Non-Seminoma Germ Cell Tumors (NSGCT) may be present as mixed (multiple histologic subtypes, which may include elements of seminoma) or pure (only one histologic subtype). Yolk Sac Tumor (YST), may secrete AFP and/or hCG (Figure 3). It often exhibits hematogenous metastasis. Choriocarcinoma always secretes hCG and never secretes AFP [2,9]. It has a poor prognosis and early hematogenous metastases occur in several locations, most often in the lungs. Embryonal Carcinoma (EC) may secrete AFP and/or hCG and has a high risk for microscopic metastases [2]. Primary testicular lymphoma is rare and is more common in men older than 60 years. It is the most commonnest bilateral tumor of the testis and also the most commonnest metastatic tumor found in the testis [20]. None of our patients carried out sperm conservation (Figure 4).
Figure 3: Distribution of patients according histological type.
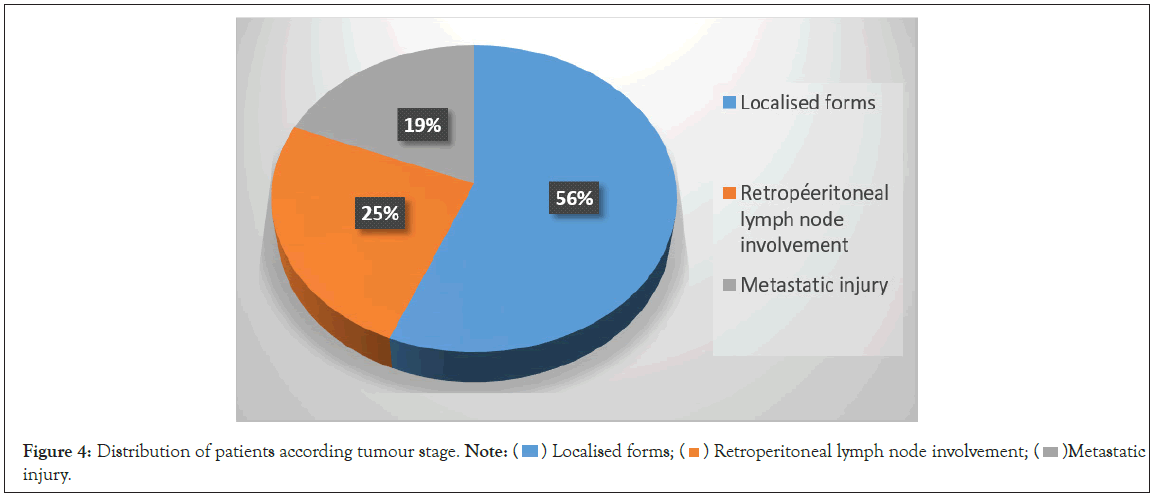
Figure 4: Distribution of patients according tumour stage. Note:  Metastatic
injury.
Metastatic
injury.
Treatment
Chemotherapy was carried out in 7 patients (43.75%). In the event of a large metastatic volume, chemotherapy will be the first treatment and orchiectomy will then be performed [9,21,22] (Figure 5).
Figure 5: Progressive appearance after orchiectomy in a patient.
Patients with CS I seminomas are typically offered 3 management options: surveillance, Radio Therapy (RT), or adjuvant chemotherapy. Seminomas are exquisitely radiosensitive. Patients with CS IIA, IIB, or IS seminoma could have an induction chemotherapy (EP 4 cycles or BEP 3 cycles) which is the preferred option for patients with IIB or IS. With this approach, cure rates are greater than 95% [9,21,22].
Patients with Non-Seminomata Germ Cell Tumor (NSGCT) CS I have 3 management options: surveillance, adjuvant chemotherapy, and Retro Peritoneal Lymph Node Dissection (RPLND) [9,10,21,22]. Patients with limited retroperitoneal disease but no metastasis outside of the retroperitoneum are categorized at CS IIA or IIB. The treatment options include RPLND or chemotherapy (BEP 3 cycles or EP 4 cycles) for those with negative STMs [9,21,22].
Evolution
Mortality was high in our study, 25% after a 6-month follow-up. This is linked to the low socio-economic level and the inaccessibility of patients to chemotherapy. In fact, in the Thies region there is no radiotherapy and chemotherapy center. However, in developed countries, the 5-year specific survival rate is greater than 90% for all stages [22,23]. Close follow-up is necessary to detect recurrences.
Testicular cancer is rare in the Thies egion of Senegal. Its management is multidisciplinary and has benefited from therapeutic advances, particularly in the field of surgery, radiotherapy and chemotherapy, making it possible to adapt the treatment to each stage. Early diagnosis and raising the technical level of reference structures could improve the prognosis of these cancers.
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
Citation: Kouka SCN, Bentefouet L, Thaim M, Faye M, Diop M, Cisse M, et al. (2023) Particularities of the Management of Testicular Cancer in the Thies Region of Senegal. Med Surg Urol. 12:334.
Received: 20-Nov-2023, Manuscript No. MSU-23-28096 ; Editor assigned: 22-Nov-2023, Pre QC No. MSU-23-28096 (PQ); Reviewed: 06-Dec-2023, QC No. MSU-23-28096 ; Revised: 14-Dec-2023, Manuscript No. MSU-23-28096 (R); Published: 22-Dec-2023 , DOI: 10.35248/2168-9857.23.12.331
Copyright: © 2023 Kouka SCN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.