Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Review Article - (2021)
Spondyloarthropathies include a group of inflammatory arthritides encompassing Ankylosing Spondylitis (AS), reactive arthritis, arthritis/spondylitis associated with Psoriatic Arthritis (PSA) and arthritis/spondylitis associated with Inflammatory Bowel Diseases (IBD). In Spondyloarthropathies, inflammation can act either by promoting atherosclerosis or by increasing the effect of conventional CV risk factors. The incidence of Cardio Vascular (CV) disease in AS is 10%-30% and includes aortic valve regurgitation, aortitis, Atrio Ventricular (AV) and/or bundle branch block. In Psoriatic Arthritis and IBD, there is increased risk of CV events, because high levels of cytokines promote atherosclerosis. Furthermore, the persistence of systemic inflammation promotes the development of myocardial inflammation.
Magnetic Resonance Imaging (MRI) can identify inflammation in the early stage of Spondyloarthropathies, which usually occurs years before the development of structural lesions. Bone Marrow Edema (BME) has been detected not only at sacroiliac joints, but also at the spine, and it is considered as the hallmark of inflammation. Cardiovascular Magnetic Resonance (CMR) allows function and tissue characterization and detects subclinical cardiac lesions, including oedema, myocarditis, diffuse subendocardial fibrosis and myocardial, despite the presence of well controlled musculoskeletal disease.
Considering that bone radiographic, cardiac echocardiographic findings and serum biomarkers are late markers of bone and heart involvement, MRI/CMR can play an indispensible role for early diagnosis follow up of bone/heart disease in Spondyloarthropathies.
Magnetic resonance imaging; Spondyloarthropathies; Bone lesions; Cardiac lesions; Heart failure
The term of spondyloarthropathies describes a group of inflammatory arthritides encompassing Ankylosing Spondylitis (AS), reactive arthritis, arthritis/spondylitis associated with psoriasis Psoriatic Arthritis and arthritis/spondylitis associated with Inflammatory Bowel Diseases (IBD). The Human Leukocyte Antigen (HLA)-B27 positivity, peripheral joint involvement mainly of the lower extremities, sacroiliitis, spondylitis, enthesitis, dactylitis, uveitis, enteric mucosal lesions and skin lesions are the classic manifestations of these diseases [1-3].
The recently developed Assessment of SpondyloArthritis International Society (ASIS) classification criteria proposed the classification of Spondyloarthropathies according to the main clinical manifestations including either predominantly axial or predominantly peripheral presentation, with or without coexisting psoriasis, IBD or preceding infection [4-8]. Inflammatory Back Pain (IBP), the leading symptom of the Spondyloarthropathies, is due to inflammation of sacroiliac joints, spine and spinal entheses. However, the sensitivity and specificity of IBP for diagnosis of axial Spondyloarthropathies is only 80% [6,7]. HLA B-27 positivity is an important index for the early diagnosis of Spondyloarthropathies. Five to 10% of the population is HLA B-27 positive. However, in patients with AS and other Spondyloarthropathies the positivity of HLA B-27 reaches 70%-95% and 70%, respectively [8].
Type of spondyloarthropathies
Ankylosing spondylitis: Ankylosing Spondylitis (AS) is the commonest expression of Spondyloarthropathies. It is 2-3 times more common in men than women. IBP in a young patient is the most frequent symptom, but peripheral arthritis and/or enthesopathy may be found in some patients. Uveitis, positive family history for AS, impaired spinal mobility or chest expansion, all supports the diagnosis of AS [1].
Axial involvement is one of the disease characteristics and 90% of patients have radiographic sacroiliitis during the course of the disease. Currently, a patient can be classified as having definite AS, if at least one clinical criterion (IBP, limitation of lumbar spine or limitation of chest expansion) plus a radiologic criterion (bilaterally grade 2 or unilateral grade 3-4 sacroiliitis) are fulfilled [9].
Axial spondyloarthritis: Unfortunately, sacroiliitis appears on plain radiographs years after the onset of IBP. Therefore, in 2009, ASAS developed two sets of criteria for classification of axial Spondyloarthropathies that include patients without definite radiographic sacroiliitis [10-14]. The new criteria include a ‘clinical arm’ and an ‘imaging arm’. The positivity of both “arms” had 82.9% sensitivity and 84.4% specificity, while the positivity of the ‘imaging arm’ alone had a sensitivity of 66.2% and specificity of 97.3%. ASAS criteria are simple and easily applicable in every day clinical practice [15].
Peripheral spondyloarthritis: After the development of ASAS criteria for axial Spondyloarthropathies, ASAS experts developed criteria for Spondyloarthropathies patients with predominant peripheral symptoms. The sensitivity of the criteria was 77.8% and the specificity was 82.2%. The new ASAS classification criteria for peripheral arthritis perform better than the previously used criteria [10].
Psoriatic arthritis: Psoriasis is a common disease affecting nearly 1%-2% of the population. In some forms of Spondyloarthropathies, concurrent psoriasis may also occur. Psoriasis may precede, occur in parallel, or appear years after the onset of arthritis. In latter cases, patients may be misdiagnosed as having other types of arthritis, such as seronegative RA or reactive arthritis; however, positive family history for psoriasis is of great help in these cases. Patients with arthritis should be also evaluated for potentially “hidden” psoriatic lesions, located under the breasts, around the umbilicus or anus, over the hairline, nasal cleft or nails [11].
Patients with psoriasis usually present inflammatory axial involvement similar to AS. However, there is several differences compared to AS including the presence of asymmetrical sacroiliitis, non-marginal syndesmophytes, asymmetrical syndesmophytes and more frequent involvement of the cervical spine [11].
Arthritis in Inflammatory bowel disease: Two types of joint involvement occur in patients with IBD including a) arthritis (inflammation) and b) arthralgia (pain without inflammation). Arthralgia is more common in IBD, occurring in 40%-50% of patients, a rate similar to that of the general population. Arthritis occurs in 15%-20% of Crohn’s Disease (CD) patients and approximately 10% of Ulcerative Colitis (UC) [12].
Approximately 60%-70% of arthritis in IBD has the characteristics of peripheral oligo-arthritis with<5 joints affected. The most commonly affected joints are the knees, ankles, wrists, elbows and hips. A smaller proportion of IBD patients have symmetrical polyarthritis of the small joints of the hands. Finally, 1%-6% of all IBD patients develop AS affecting the sacroiliac joints and the spine. While large joint arthritis is almost always associated with active IBD, AS and small joint polyarthritis appear independently of the patient’s IBD [12].
Ankylosing spondylitis: The Incidence of Cardiovascular (CV) involvement in AS ranges between 10% to 30% and may presented as aortic valve regurgitation, aortitis of the ascending aorta, Atrio Ventricular (AV) and/or bundle branch block [13]. The most frequently observed complications are conduction defects and aortic regurgitation. Mitral regurgitation in AS is rare, but may lead to Heart Failure (HF) [14-17]. CVD is more frequent in patients with long-term AS and peripheral joint involvement [18].
The incidence of aortic dissection has been reported about 2.9 in 100,000 and represents the most fatal complication of the disease [19,20]. The most frequently observed symptom in AS patients is chest pain; however, it may have atypical findings including lack of pain or development of cardiac, neurological symptoms or evidence of ischemic extremities [21,22]. Aortic dissection is generally observed between the ages of 50-70 years and the ratio of men to women is 2:1 [21].
The main sources of stroke in AS are the lesions of the proximal aorta, but paraplegia may develop, due to distal lesions involving the spinal arteries. Although stroke and paraplegia develop rarely, their incidence in cases of aortic dissection is ranged from 2% to 8% [23].
Psoriatic arthritis: Psoriatic Arthritis has an increase in risk of clinical and subclinical CVD, mainly due to accelerating atherosclerosis. Both conventional and nonconventional CV risk factors contribute to increase atherosclerosis with consequent increase of CV risk. Although conventional risk factors occur more frequently in patients with Psoriatic Arthritis, they can only partially explain the excess of CV risk. It seems that inflammation is an important precipitating factor for the increased CV risk in Psoriatic Arthritis. Inflammation can act either by directly promoting the development of atherosclerosis or by increasing the effect of established conventional CV risk factors [24]. Further more systemic inflammation can also precipitate myocardial inflammation [24].
Inflammatory bowel disease: Patients with IBD are at increased risk of CV events. High levels of cytokines, C-Reactive Protein (CRP) and homocysteine in IBD may contribute to endothelial dysfunction finally leading to atherosclerosis. Although IBD patients do not present the typical risk factors for CVD, changes in lipid profiles similar to those seen in patients with CV events have been reported. Furthermore, increased levels of coagulation factors, frequently occurring in IBD, may predispose to arterial thromboembolic events. Finally, the gut itself may have an impact on atherogenesis through its microbiota. Microbial products are released from the inflamed intestinal mucosa into the circulation through a leaky barrier. As a consequence, the increase of proinflammatory cytokines contributes to endothelial damage and CV events [25].
There is a link between bone inflammation in Spondyloarthropathies and CVD development which was usually attributed to Coronary Artery Disease (CAD). However, the commonest CVD expression in Spondyloarthropathies is Heart Failure (HF), which may start at diagnosis and increases proportionally to duration and severity of the systemic inflammation. However, it should be noted that HF in Spondyloarthropathies is not the result of epicardial coronary artery disease, but is related to myocardial inflammation, coronary microvascular dysfunction and fibrosis, all of them leading to Heart Failure with Preserved Ejection Fraction (HFPEF) [26].
Pro-inflammatory mediating factors that are characteristic of systemic inflammation such as leptin, aldosterone and neprilysin can cause expansion and biological transformation of Epicardial Adipose Tissue (EAT) leading to coronary microvascular injury and myocardial fibrosis [27]. In patients with expanded EAT, the release of leptin and aldosterone promote myocardial inflammation, microcirculatory dysfunction and fibrosis [27]. These effects are opposed to the antiinflammatory and anti-fibrotic actions of endogenous natriuretic peptides and lead to impairment in the distensibility of the Left Ventricle (LV) [28]. Therefore, blood volume is regulated only due to an increase in cardiac filling pressures. This finally leads to exertional dyspnoea and HF. EAT volume is increased in Spondyloarthropathies and 30%-50% of affected patients have subclinical cardiac inflammation, microcirculatory dysfunction and fibrosis finally leading to HFPEF.
Spondyloarthropathies patients are characterized by increased levels of aldosterone in blood and inflamed tissues and therefore, spironolactone, an aldosterone antagonist, has been proposed as a treatment for these arthritides [29]. At the same time, the increased levels of leptin in blood and synovial fluid, found in Spondyloarthropathies, are associated with the extent of joint/bone involvement and linked with patient-reported symptoms and disease activity [30]. Interestingly, high levels of leptin may identify Spondyloarthropathies patients, who respond poorly to anti-inflammatory treatment [31].
The currently used anti-inflammatory agents in the treatment of Spondyloarthropathies have the potential to reduce the risk of cardiac involvement and HFPEF [32]. In addition, it is possible that drugs modulating the leptin-aldosterone-neprilysin axis could modify the CV consequences of systemic inflammation and change the clinical course of HFPEF in these patients [33].
Imaging bone and heart involvement in spondyloarthropathies
The indispensible role of magnetic resonance imaging: The pathophysiologic mechanisms, already discussed, make clear the correlation between the systemic inflammation and the development of CVD and emphasize the need for a concurrent assessment of both bone and cardiac inflammation. In this context, the role of a sensitive and reproducible imaging modality that can identify early the presence of inflammation is of great value for both bones and heart. The role of various modalities in the early inflammation identification in bones and heart is described below.
Role of imaging for bone/joint assessment in Spondyloarthropathies: The imaging of the sacroiliac joints and the spine plays an important role in the diagnosis and monitoring of Spondyloarthropathies. Sacroiliitis on conventional radiography was used as an important criterion for AS diagnosis. Usually, bilateral grade ≥ 2 or unilateral grade ≥ 3 sacroiliitis are considered typical for the diagnosis of AS [13]. However, radiographic sacroiliitis reflects changes late in the course of the disease and only in a subgroup of Spondyloarthropathies patients [12].
The introduction of Magnetic Resonance Imaging (MRI) in the clinical practice has changed dramatically the diagnostic algorithm of Spondyloarthropathies, because MRI-detected sacroiliitis has been included in the new Assessment of Spondylo Arthritis Society (ASAS) classification criteria for axial Spondyloarthropathies, as a basic diagnostic tool [34]. Prior to this innovation, radiological sacroiliitis was diagnosed on the basis of x-rays, where changes can be detected only at advanced stages of the disease and therefore, they have low specificity for patients in early stages of the disease. In contrast, MRI can identify inflammation in the early stage of Spondyloarthropathies, which usually occurs years before the development of structural lesions. Bone Marrow Edema (BME) has been detected not only at sacroiliac joints in axial Spondyloarthropathies, but also at the spine, and it is considered as the hallmark of inflammation (Figures 1 and 2). Among inflammatory lesions that can be detected on MRI at the sacroiliac joints, only BME has been considered as a reliable index in the identification of active sacroiliitis [35]. This finding is thought to reflect the presence of osteitis and correlates with histologically documented inflammation. Bollow, et al. demonstrated an inflammatory infiltrate of T-cells, macrophages and scarce B-cells in biopsy of patients with MRI-detected sacroiliitis [35,36]. Moreover, they found a higher number of inflammatory cells in patients with active sacroiliitis on MRI, but not in patients with chronic sacroiliitis. Recent studies support that not only the presence, but also the severity of BME is important for the disease diagnosis. In a prospective study of 40 patients with IBP, the presence of severe sacroiliitis in association with HLA-B27 positivity was highly predictive of a diagnosis of AS at 8 years, with a positive likelihood ratio of 8.0. In contrast, patients with mild or no BME had a low likelihood to develop AS [37]. In another study comparing patients with IBP with patients with mechanical back pain or healthy volunteers, BME of the sacroiliac joints was found also in the control group, although it was less frequent compared to patients with IBP. The very early IBP of Spondyloarthropathies can be differentiated from non-Spondyloarthropathies, based on BME severity [38].
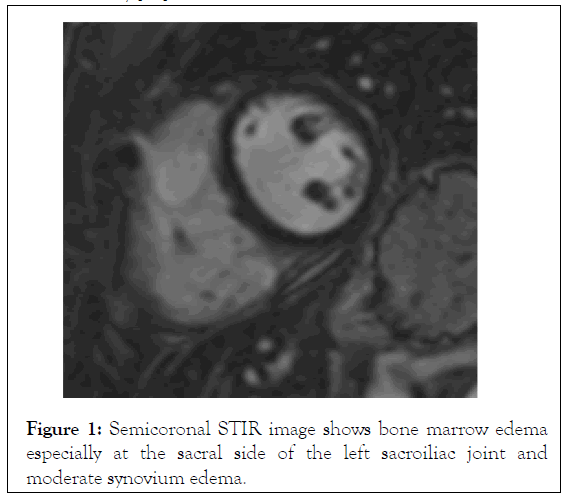
Figure 1: Semicoronal STIR image shows bone marrow edema especially at the sacral side of the left sacroiliac joint and moderate synovium edema.
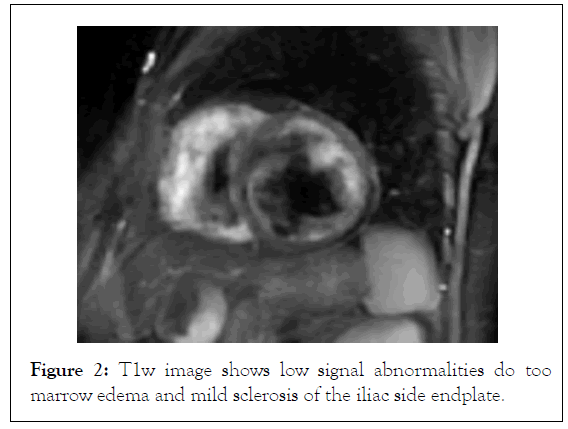
Figure 2: T1w image shows low signal abnormalities do too marrow edema and mild sclerosis of the iliac side endplate.
MRI can identify active inflammation at sacroiliac joints and spine in established or early axial disease, independently of disease stage [10]. ASAS classification criteria for axial Spondyloarthropathies have 2 arms: An imaging and a clinical arm. The imaging arm includes either sacroiliitis on conventional radiography or on MRI, which is of indispensible value for the recognition of pre-radiographic changes in early Spondyloarthropathies [4]. Regarding spondylitis, which may also occur before sacroiliitis, the diagnosis of a “positive MRI” for spinal inflammation is also needed [39]. However, there are insufficient data regarding the use of spinal MRI and the specificity of spinal findings in the axial Spondyloarthropathies diagnosis [40].
Active inflammatory lesions (bone marrow edema, osteitis, synovitis, enthe-sitis, capsulitis) and structural damage (sclerosis, erosions, fat deposition, ankylosis) can be detected by MRI. ASAS/OMERACT imaging group defined that a minimum amount of BME in one lesion of at least two adjacent slices or more than one lesion at least in one slice is required for the definitive diagnosis of sacroiliitis [41].
MRI can also assess the evolution of spinal damage of AS. Usually, it begins with bone inflammation that may be visible as osteitis, typically seen on a Short Tau Inversion Recovery (STIRT2) image of the MRI [42,43]. To repair the tissue, subchondral bone marrow is replaced with fatty metaplasia, which presents as a fatty lesion on a T1 weighted MRI scan [42,43]. Inflammation or intermediate stage fatty lesions may play an important role in the development of new bone formation (structural damage), emphasizing the need for early deterioration of inflammation to reduce risk of further bone damage [43]. Almost 60% to 70% of AS patients develop irreversible structural changes leading in spinal fusion and reduced spinal mobility [43,44]. Although AS patients usually present reduced physical/spinal function, due to structural lesions and inflammation, in patients with non-radiologic positive Spondyloarthropathies (nr-axSpondyloarthropathies), this functional disability is typically due to inflammation alone [44]. Risk factors for progression of spinal disease in AS patients include male sex, presence of syndesmophytes at baseline, elevation of CRP and smoking (in men) [45].
Recently, several studies have examined the MRI characteristics of new bone formation in axial skeleton in patients with Spondyloarthropathies and assessed findings that showed potential markers of disease progression [46-52]. The intraarticular high signal intensity on T1-weighted MR images known as “Backfill” with high signal intensity similar to adipose tissue on T1-weighted MR images in the sacroiliac joint Spondyloarthropathiesce was considered as a metaplastic tissue refilling of the eroded subchondral bone [46,50,51]. There is no consensus about the clinical value of this finding, because until now there is no histopathological evaluation of this lesion [50]. However, this intra-articular high signal intensity on T1- weighted MRI images of the sacroiliac joints has been documented in 38%-63% of Spondyloarthropathies patients ≤ 45 years old and had a high diagnostic value for this disease [46,50]. There is also supported that this finding should be considered stronger that the classic ASAS criteria for Spondyloarthropathies sacroiliitis, even if there is no concurrent BME [51].
Ankylosis of the sacroiliac joints is the typical finding of endstage axial Spondyloarthropathies. This may appear as low signal intensity obliteration of articular cortical margins, on most MRI sequences, but it may present high signal intensity on T1- weighted MR images, when the subarticular bone marrow crossing the sacroiliac joint has high-fat content [50,51].
The presence of discal high signal intensity on T1-weighted MR images, which has only been evaluated in a limited number of patients, has high signal intensity similar to adipose tissue on T1-weighted images within the intervertebral disc and was considered as an early discal calcification [52-54]. In a recent study, it was considered as highly specific for Spondyloarthropathies, although it needs further validation [52].
Syndesmophyte formation is defined as bony growth originating from the Sharpey fibers of the annulus fibrosus [52]. On sagittal spinal MRI, syndesmophytes will be observed as longitudinal bony outgrowths at the anterior and posterior corners of the vertebral bodies, oriented craniocaudally. The signal intensity on T1-weighted images is isointense or hyperintense to red bone marrow in case of presence of fatty bone marrow [52]. However, non-bridging syndesmophytes in the absence of other findings of new bone formation were only seen in patients without Spondyloarthropathies [21] and therefore, they should not be used for Spondyloarthropathies diagnosis or follow up. Vertebral corner bridging also known as “bridging syndesmophytes” or “ankylosis within the annulus fibrosus” is observed in the anterior or posterior corners of the vertebral bodies, at the Sharpey fibers of the annulus fibrosus of the intervertebral disc [52]. The signal intensity on T1-weighted images is isointense or hyperintense to red bone marrow, in case of fatty bone marrow [52]. In contrast to non-bridging syndesmophytes, this MRI feature is specific for Spondyloarthropathies [52].
Transdiscal ankylosis, also known as “non-corner ankylosis”, is defined as bone fusion crossing the vertebral joint Spondyloarthropathiesce through the expected location of the nucleus pulposus in the intervertebral disc, with obliteration of the cortical margins of the vertebral body [52]. Similar to syndesmophytes, the signal intensity on T1-weighted images is isointense or hyperintense to red bone marrow, in case of presence of fatty bone marrow [52]. This finding is specific for axial Spondyloarthropathies [52], and is considered a marker of late disease, because axial Spondyloarthropathies almost always starts in the sacroiliac joints, typically leaving the spine unaffected for long time. However, it may also be found in younger patients with more severe disease [52].
Role of imaging for cardiac assessment in spondyloarthropathies
The main imaging modalities used for evaluation of myocardial status in Spondyloarthropathies include echocardiography and Cardiovascular Magnetic Resonance (CMR). Subclinical cardiac dysfunction was identified in AS patients despite well controlled musculo-skeletal disease, using echocardiography. Furthermore, aortic stiffness and Left Ventricular Global Longitudinal Strain (LVGLS) were increased in AS patients. In another echocardiographic study, early, subclinical myocardial dysfunction was observed in Inflammatory Joint Disease (IJD) patients with preserved left-ventricular ejection fraction, but without traditional CV risk factors. In these patients, disease activity was the main predictor of myocardial strain impairment. In another study, it was documented that AS patients had lower LVGLS compared with controls, independently of confounders. Furthermore, lower LVGLS was associated with larger aortic root diameter. Finally, most asymptomatic Spondyloarthropathies patients have concentric LV remodeling, which is closely associated with subclinical Left Ventricular Systolic Dysfunction (LVSD). Echocardiography, although it is an easy, low cost and widely available modality, it has the limitations of operator and acoustic window dependency and cannot perform cardiac tissue characterization.
In contrast to echocardiography, CMR is operator and acoustic window independent and can provide function and tissue characterization information noninvasively in the same examination. A CMR study, including AS patients with abnormal findings on screening echocardiography, identified that aortic arch Pulse Wave Velocity (PWV) was significantly higher in the AS group compared with the controls. Higher PWV in the aortic arch was associated with functional disability, the presence of non-ischemic hyperenhancement and reduced LV systolic function. In another CMR study in AS patients, global LV dysfunction and focal areas of hyperenhancement was identified. Myocardial Extra Cellular Volume Fraction (ECV) was associated with the degree of disease activity. Furthermore, occult CMR lesions, including oedema, myocarditis (Figures 3 and 4), diffuse subendocardial fibrosis and myocardial infarction are not unusual in treatment naïve Spondyloarthropathies patients and may be reversed with appropriate treatment. Recently, anti-TNF treatment, applied in Spondyloarthropathies patients, reduced subclinical myocardial inflammation and improved CV function proving that CMR may have a place to assess CV disease progression and response to treatment.
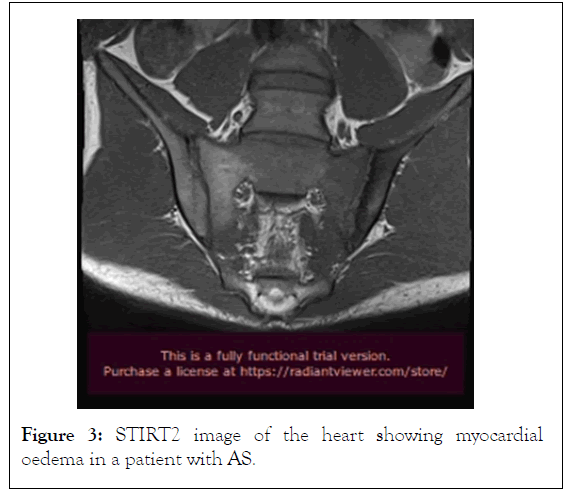
Figure 3: STIRT2 image of the heart showing myocardial oedema in a patient with AS.
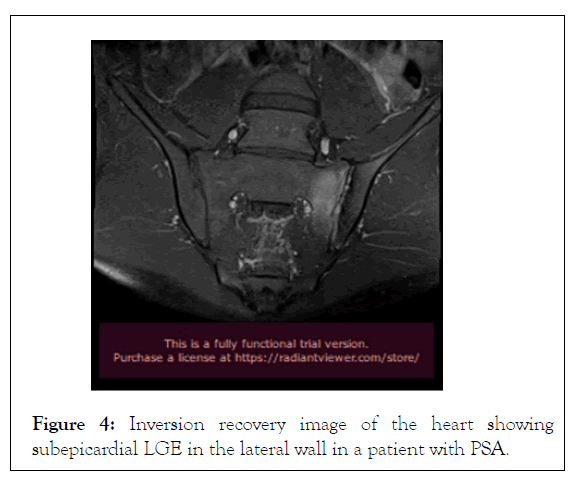
Figure 4: Inversion recovery image of the heart showing subepicardial LGE in the lateral wall in a patient with PSA.
MRI vs. biomarkers in spondyloarthropathies
The evaluation of biomarkers in Spondyloarthropathies is of particular interest, because the two most commonly used biomarkers, Erythrocyte Sedimentation Rate (ESR) and CReactive Protein (CRP), are of very low sensitivity and specificity. The second reason is the need for cost-effectiveness assessment after treatment of Spondyloarthropathies patients with the very expensive tumor necrosis factor-alpha (TNF-alpha) blockers. In these cases, the clinicians need to be much more accurate in predicting disease progression, evaluating disease activity and monitoring therapeutic efficacy. Furthermore, according to the results from the SpondyloArthritis Caught Early (Spondyloarthropathiesce) cohort the disease process in axial spondyloarthritis is not reflected by alterations in blood inflammatory indices such as CRP, ESR and Calprotectin. Additionally, serum levels of Interleukin-27 (IL-27), human β- Defensin-2 (hBD-2) and Lipcolin-2 (LCN-2) were also not elevated. In contrast, the direct visualization of the inflammatory process might be a more successful approach to identify imaging biomarkers for axial Spondyloarthropathies.
Regarding the cardiac evaluation, troponin and BNP are the most commonly used biomarkers. In the appropriate patient, an elevated troponin indicates heart damage and should prompt urgent inpatient investigations, while a normal troponin indicates low risk, but not absence of risk. Furthermore, troponins may be falsely elevated in certain situations and do not always indicate heart attack. These situations include myocarditis, Takotsubo syndrome, pulmonary embolism and trauma including also cardioversion or defibrillation. Finally, the prevalence of an increased troponin T in biopsy-proven myocarditis is only 35%-45%.
More specifically in patients with axial Spondyloarthropathies, both reduced Longitudinal Strain (LS) and elevated serum Highsensitivity Troponin I (hsTnI) are promising independent predictors for CV events and those with LS ≥ 17.5% and hsTnI ≥ 3.0 pg/ml are at the highest risk of CV events.
It is impressive that although MRI has been used very early in the assessment of bone/joints, its application on cardiac evaluation of Spondyloarthropathies patients has been only recently applied. There are many reasons for this including:
• At the very first moment of its application MRI was considered as the ideal tool to assess bones and soft tissues. Therefore, it was introduced very early in the diagnostic algorithm of Spondyloarthropathies [34].
• Cardiovascular Magnetic Resonance (CMR) is a more over demanding application that has become only recently available to cardiologists. Additionally, cardiologists are “obsessed” with echocardiography, which is a modality unsuitable to detect myocardial inflammation, because it cannot perform tissue characterization.
Under these circumstances, only parameters available by echocardiography such as aortic dilatation or diastolic dysfunction were considered as serious cardiac complications in Spondyloarthropathies. Furthermore, although HFPEF is the main cause of CV death in Spondyloarthropathies, information regarding acute myocardial inflammation/fibrosis was completely ignored, due to the inability of echocardiography to detect these phenomena. Finally, the knowledge that CV involvement may exist before or at diagnosis of various Spondyloarthropathies, suggests that CMR should be considered in the diagnostic algorithm of CV assessment of Spondyloarthropathies. At the moment published literature regarding CMR in Spondyloarthropathies is poor. However, the need to assess myocardial inflammation/fibrosis as an effort to prevent the development of HFPEF in Spondyloarthropathies patients should motivate further multicenter studies to establish the role of CMR in the Spondyloarthropathies guidelines for CV evaluation.
Inflammation is the fundamental pathophysiologic background of bone/heart lesions in Spondyloarthropathies patients. Since blood inflammatory indices are of limited value to assess bone/ heart disease activity, there is a tremendous need for a targeted imaging approach. Bone MRI has been already included in the diagnostic algorithm of Spondyloarthropathies, but the assessment of myocardial inflammation/fibrosis, using CMR, is still not included in the routine evaluation of Spondyloarthropathies. However, the fact that HFPEF represents the main cause of CV mortality in Spondyloarthropathies, suggests that a reconsideration of cardiac evaluation in Spondyloarthropathies is needed.
Citation: Mavrogeni SI, Nikas I, Bonou M, Kitas GD (2021) Pathophysiology of Bone and Cardiac Involvement in Spondyloarthropathies: The Indispensible Value of Magnetic Resonance Imaging. Intern Med. S8 :004.
Received: 23-Sep-2021 Accepted: 07-Oct-2021 Published: 14-Oct-2021 , DOI: 10.35248/2165-8048.21.s8.004
Copyright: © 2021 Mavrogeni SI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.