Journal of Down Syndrome & Chromosome Abnormalities
Open Access
ISSN: 2472-1115
ISSN: 2472-1115
Research Article - (2017) Volume 3, Issue 2
The explanations for the motor learning deficits observed in individuals with Down Syndrome (DS) emphasize a complex interplay among the structural and functional body abnormalities of this population. To further comprehend how this pathological condition functions, researchers are now attempting to understand how visual and motor systems process information and how this processing correlates with the development of motor learning in individuals with DS. The purpose of the current study was to investigate whether individuals with DS are able to perceive and differentiate between visually presented sequences of biological motion that portrayed walking at different speeds. We tested 17 participants with DS and 17 typical develop individuals (CG) who were matched for age and gender on a task requiring subjects to make fine discriminations between biological motion stimuli in the form of point-lightwalkers that walked at different speeds. Individuals with DS were unable to recognize differences in walking speeds between walkers, and also demonstrated slower reaction times on the task than the CG. The CG was able to perform the task well above chance level and faster than DS subjects. These results shed light on the biological motion processing capabilities of people with DS, and provide insights for future research in this field.
<Keywords: Speed perception; Down syndrome; Point-Light-Walkers; Intellectual disabilities; Visuo-motor integration; Biological motion
Action and perception are two inherently linked aspects of human behavior that allow us to understand and interact with our surroundings. The interplay between action production and perception enables humans to act in a number of highly demanding situation such as unusual, novel or unstable environments [1]. Babies interact with their environment in a myriad of different ways such as imitation, selfmotion and observing the examples of others [2-4]. These interactions serve to shape not only their knowledge about the world, but also their understanding of the functionality of their bodies.
Action performance interacts with action perception, and viceversa. This is reflected in the brain, as both performing and perceiving actions activate similar cortical areas [5]. Previous research has revealed significant interactions between action perception and action execution, and studies have shown that action perception can interfere with action execution reciprocally [6-8]. It is particularly insightful to study such interactions in populations with abnormal skeleto-muscular and/or neural development, as in these populations the brain may have adapted differently to handle physical differences, which in turn may influence how affected individuals interact with their surroundings. For example Sinha et al. [9] have shown that individuals with autism spectrum disorder, adapt their sensory input atypically, resulting in diverse behavioral traits.
Down Syndrome (DS) is one of the most common genetic disorders (14 per 10 000 live births) caused by the presence of a complete or partial third copy of chromosome 21 (Trisomy 21) [10]. DS is a genetic change that impacts not only intellectual ability, but also motor development and motor control over the lifetime [11,12]. Abnormal brain functioning related to learning has been reveled in a number of studies [13-16]. In addition to neurodevelopmental abnormalities, corporal abnormalities are also common in DS. These abnormalities are highly related to the impaired motor control of voluntary movements [11,17].
Children with DS require more time to learn basic movements. Additionally, as movement complexity increases they achieve gross motor functions at an average age that is almost twice that of typically developed children [18]. However, despite delays in motor development and the control of voluntary movements, the DS population does not exhibit a complete absence of the production of gross motor skills. For instance, individuals with DS are often capable of walking and jumping, as well as fine motor skills and manual dexterity. Examples of functional motor development can be seen when special teaching strategies are implemented [19-24]. This demonstrates that individuals with DS are at least somewhat able to adapt to their motor impairments. In more realistic tasks, Latash [11,17] argue that, when confronted with an unexpected or unclear motion situation, individuals with DS choose to act slowly (bradykinesia) during the initiation phase of movement, in order to procedure safe, rather than fast movements.
Although motor deficits have been studied extensively in the DS population, relatively little research has focused on how visual information is integrated during motor activity and whether motor deficits also impact the visual system, and vice-versa. Interactions between the visual and motor systems may play an important role in motor learning, specifically when visually perceiving the actions of others [25-27]. Interestingly, children with DS are generally as good as, if not better than, typically developed children at imitation based tasks [28]. Despite this, evidence exists to suggest that children with DS exhibit deficiencies in coupling visual information to motor output [29]. Research by Virji-Babul et al. [30] suggests that individuals with DS demonstrate impairments when combining visual and motor information. For example, when stepping over an object child with DS exhibited reduced step length variability compared to typically developed controls and tended to stop in front of objects before stepping over them. It was suggested that this may reflect difficulties in adjusting the motor output based on the visual input.
One approach to investigating visuomotor coupling in DS is to study the ability of individuals with DS to discriminate between visual representations of motor behaviors. This is frequently achieved in typically developed individuals by using point-light-walkers (PLW), a class of biological motion stimuli created by attaching points of light to the joints of an actor while they are recorded performing an action [31]. Typically developed subjects are able to obtain a wealth of information from PLW displays, such as gender [32], emotion [33] and even a person’s identity [32,34]. Previous research has used PLWs to study the visual processing of biological motion in DS. Virji-Babul et al. [29] have shown that individuals with DS perform worse than controls in tasks requiring the discrimination of PLWs from non-biological shapes and in the discrimination of emotion from PLWs. Riddell et al. [35] have shown that these deficits likely stem from an inability to integrate global configural cues present in the PLW stimulus.
The aim of the current study was to further investigate the perception of PLW stimuli in individuals with DS. Specifically, we were interested in assessing whether individuals with DS are able to make fine discriminations between PLWs performing the same actions at different speeds. Discrimination between subtle differences in visually perceived actions is a behaviorally relevant task that has ramifications for daily functioning. Additionally, we were also interested in reaction times on the task, as reaction times may indirectly reflect the outcome of the visuomotor processing [36,37]. Based on previous research showing that PLW perception is impaired, but not completely absent in individuals with DS [29,35], we hypothesized that in a behaviorally relevant task requiring subjects to compare PLW speeds, performance should be similar between the DS and control groups when differences in PLW walking speeds were relatively large. When the difference between the PLW speeds was small, however, we expected the control group to outperform the group with DS. Based on already published literature about reaction time in DS, Davis et al. [38], Galli et al. [39], Anson [40]. We also hypothesized, that the reaction time performance on our study would be significantly different between the groups.
Participants
Control and DS groups were matched for gender and age. The DS Group consisted of 17 participants, 10 females and 7 males, (M=21.47, SD=6.56 years), and the Control Group (CG) consisted in 17 typical develop participants, 10 females and 7 males, (M=21.18, SD=6.32 years). Control participants had no history of mental illness or physical disability. All non-adult subjects from CG were in either primary or high school at the time of testing. All of participants with DS attended schools for people with special needs, or were enrolled at institutions that specialized in teaching adults with developmental disorders. All participants had normal or corrected to normal visual acuity, with no history of eye surgery. Written informed consent was obtained from both participants and their guardians or parents. The experimental design was approved by the local ethics committee of Westf¨alische Wilhelms-Universit¨at M¨unster, (06-2016-JWTC-BM).
Apparatus
For the behavioral task, the Stimuli were produced using Matlab (Mathworks) with the PsychophysicsToolbox (version 3) add-on [6,41]. The Stimuli were generated and presented using a laptop 15.4” Apple MacBook Pro (2 Gb RAM, 2.4 Ghz) equipped with an Nvidia GeForce 9400M graphics card (256 Mb). The laptop display operated at a resolution of 1440 × 900 pixels with a refresh rate of 60 Hz.
Stimuli
The PLW were produced by placing single white points at the left and right ankle, knee hip, shoulder, elbow, and wrist joints (Figure 1). Kinematic data for PLW generation was obtained using a MotionStar Wireless motion capture system (Ascension Technology Corp., Burlington, USA). Data was gathered from 11 typical develop adults (4 female, 7 male) who were instructed to walk at a natural pace during recording. We distributed all the 11 obtained walkers through the whole experiment randomly.
Figure 1: Depiction of an example PLW. The connections between the joints are highlighted in purple to assist the reader in recognizing the walker’s underlying form. These connections were not present during the experiments.
All walkers contributed almost equally to the dataset. Consequently, each subject at least saw 6 different obtained PLWs. Individual PLW points subtended 0.1 degrees of visual angle and had a luminance of 75.34 cd/m2. Because the kinematic data for walkers was obtained from different individuals the maximum stride width and walker size varied between walkers. The largest walker in the set subtended a maximum of 4.30 × 9.05 degrees, while the smallest subtended a maximum of 1.91 × 5.25 degrees. Participants in our experiment did not report that any walker appeared to be easier or more difficult to detect, this was also reflected in the data. Walkers were always presented centrally against a black background, which had a luminance of 2.47 cd/m2 (Figure 1).
Procedure
Participants were seated at a computer with their hands on the keyboard. The stimuli consisted of the successive presentation of two PLWs, each with duration of 1600 ms and with an interstimulus interval of 500 ms. after the second walker disappeared the participants were asked to choose which of the PLWs was faster. Participants responded by pressing either the right shift key if they perceived the first walker as walking faster or left shift key if they perceived the second PLW to be walking faster. The number of correct responses and reaction times were recorded. Reaction times were recorded from the offset of the second stimulus. After the participant pressed a button a new stimulus was presented. This sequence was repeated 40 times. Two walker speed conditions were presented, in one condition the slower of the two walkers walked at 3 km/h while the faster walker walked at 5 km/h and in the other condition the slower walker walked at 4 km/h while the faster walker walked at 5 km/h. Speed conditions were randomly interleaved and the presentation order of the faster/slower walker was also randomized.
Prior to the main experiment, participants with DS performed a control experiment to ensure that individuals with DS were not impaired in more generalized motion detection. A sample of 9 DS subjects, 3 female and 6 male, age M=17.22, SD=3.11, viewed spatially scrambled and normal PLWs that translated horizontally right or left across the computer screen, their task was to judge the direction of motion. Data and methods from this control experiment have been published previously in another study of our group [35].
Participants completed several practice trials to ensure that they could spontaneously perceive a person or not from the PLW stimuli and that they understood the task. If a participant could not meet either of these two conditions during practice they were excluded from the experiment. Eight subjects from the initial DS subject pool did not recognize PLWs and were thus excluded from the experiment. To measure visual acuity we performed an examination using Snellen Eye Test (LongLife TM Project-O-Chart, Reichert Inc, Depew, NY) adjusted for a 6.1 meter viewing distance for each participant according to a standard protocol, the test was performed in the same room and under the same lighting conditions that the subjects executed the experiment.
We measured and compared reaction times (RT) and number of correct responses between the groups. These parameters were measured in the two walker speed conditions, (3-5 km/h and 4-5 km/h). The analysis of the number of correct answers and RT data were performed using SPSS (IBM, USA) and R (R Development Core Team, 2013) with the lme4 package [42].
Statistics
For the control experiment we scanned the data for outliers. A single participant produced responses more than three standard deviations lower than the mean of the group and was therefore omitted from the analysis. The control experiment data also failed to meet the assumption of normality. Comparisons were therefore carried out using non-parametric tests. A binomial test was used to compare performance against the 50% correct responses threshold that defined chance level. A binomial linear mixed model with participant included as a random factor and walker type as a predictor was implemented to differentiate performance in the regular and scrambled PLW conditions. Satterthwaite approximation [43] was used to obtain p values for the fixed effects of the models.
To assess difference in the main experiment between the number of correct responses in the DS and control groups we used a binomial generalized linear mixed model with subject as a random effect, and walker speed (3-5 km/h and 4-5 km/h) and group (control/DS) as fixed effects. Satterthwaite approximation [43] was used to obtain p values for the fixed effects of the models. A mixed ANOVA with walker speed as a within subjects factor and group as a between subjects factor was used to assess differences in transformed reaction speeds between the two groups.
Prior to statistical analysis reaction times (RT) were transformed to reaction speeds by taking the inverse of the reaction time (1/RT) [44]. This was done to correct for the skewed distribution of reaction times. The data for the number of correct responses (out of 20 for each walker speed condition, out of 40 in total) was distributed normally.
In the control experiment, participants gave a median of 39.50 out of 40 (Range=38-40) correct responses. This performance level was significantly higher than chance level (p<0.001, Z=69.87). The number of correct responses did not differ for normal PLWs and scrambled PLWs (β=-0.41, SE=0.92, Z=0.45, p=0.065).
We investigated the ability of typically developed and DS individuals to perform fine discriminations between PLWs walking at either, 3 km/h and 5 km/h or 4 km/h and 5 km/h. The CG was able to reliably discriminate between the walkers in both speed conditions, while performance in the DS group was close to chance level in both conditions (Figure 2).
Figure 2: Mean number of correct responses for each condition (out of 20) made by DS and control participants. White bars denote performance when the slower of the two presented walkers walked at 3-5 km/h, while gray bars show performance when the slower walker walked at 4-5 km/h. Vertical lines represent 95% confidence intervals. The dashed horizontal line denotes chance performance.
The difference in the numbers of correct answers between the DS and CG groups was significant (β=2:24, SE=0.032, Z=7:02, p<0.01), with DS subjects (M=11.74, SD=3.60) performing significantly worse than controls (M=19.00, SD=1.44). There was also a significant interaction between walker speed and group (^=1.54, SE=0.50, Z=3.16, p<0.01), with CG, but not DS subjects, performing better when the difference between walker speeds was larger (3-5 km/h). Tukey adjusted post-hoc tests were used to further investigate the interaction. No significant difference was found between the 3-5 km/h and 4-5 km/h conditions for DS subjects (p=0.94). Control subjects performance, on the other hand, did differ significantly in the two speed conditions (p=0.002).
One sample t-tests were used to compare performance in the DS and control groups against chance level performance, which was defined as 50% correct responses. Holm adjustments were applied to p values to control for type one error rates [45]. No significant differences were found between the performance of subjects with DS for either the 3 km/h walker condition (t (16)=1.99, p=0.13) or the 4 km/h condition (t (16)=1.96, p=0.13). Control participants, on the other hand, differed significantly from chance in both conditions (3 km/h: t (16)=65.60, p<0.01, 4 km/h: t (16)=19.91, p<0.01.
We also assessed differences in reaction speeds between the two groups. We found that DS and control subjects differed significantly in their reaction speeds (F (1,32)=29.50. p<01, 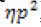 =0.48), but there was no significant main effect of walker speed (F (1,32)=0.005, p<0.94) or interaction (F (1,32)=0.01 p<0.99). Control subjects were significantly faster than subjects with DS (untransformed reaction times are shown in Figure 3).
=0.48), but there was no significant main effect of walker speed (F (1,32)=0.005, p<0.94) or interaction (F (1,32)=0.01 p<0.99). Control subjects were significantly faster than subjects with DS (untransformed reaction times are shown in Figure 3).
In the current study we investigated DS and control subjects’ ability to make fine discriminations between PLWs walking at different speeds. Results from our experiment show that individuals with DS were not able to discriminate between PLWs at any of the measured walking speed levels. In comparison the CG performed the task with ease, but performance decreased as differences between the test and reference walker were reduced. These results are somewhat in line with our hypotheses. We initially predicted that when the difference between PLW speeds was large (3-5 km/h) the performance of the groups should be equal with differences occurring as the differences between the walking speeds were reduced thus making the task more difficult. We found, however, that performance was impaired at all levels measured in the current experiment for the DS group. For DS subjects, performance remained around chance level even when differences between the presented walking speeds were relatively large and control subjects were able to perform the task at near-ceiling level. This implies that DS subjects were unable to reliably discriminate between the stimuli and that this deficit was not dependent on the difficulty of the task, at least at the levels presented in the current experiment. These results indicate that deficits in individuals with DS are not limited to cognitive or motor tasks, as is frequently reported in the literature [11,17,46,47], but also extend to processing and perceptual tasks that require recognition and fine discrimination of actions.
In addition, we hypothesized that reaction times would differ between the two groups; this was confirmed by the experimental data. However, we cannot rule out the possibility that CG subjects also anticipated their responses. Short reaction times in the CG, M=95 SD=10 ms, could be seen as evidence of this. This phenomenon was not observed in the DS group, who demonstrated distinctly longer reaction times. Delayed reaction times have been reported in DS for a number of stimuli such a light, sound, and combinations light/sound signals [38]. We suggest that these results could represent delays in the neurological process underpinning biological motion processing in DS; however, we also emphasize that other factors, such as a more generalized motor slowing [39,40] could have also produced these results. As such, we are hesitant to suggest that the increased reaction times in the current experiment are a definitive indicator of impaired biological motion processing in DS in the current experiment. Nonetheless, the slowed reaction times reported here are accompanied by severely reduced task performance, and we suggest that disentangling these effects may provide further insights into the processing of biological motion in DS.
A potential explanation for the current results could stem from the fact that motor milestones develop later in DS [48]. The perception of PLWs can be affected by learning [49,50]. Because people with DS generally take a longer time to initiate walking, this may interfere with their perception of PLW and their ability to perform fine discriminations actions such as walking [51]. On the other hand, walking is an extremely common and highly practiced activity for both normal and DS populations. Thus, it is also possible that the deficits observed in the current study are not directly correlated with motor learning, and may potentially arise from perceptual or processing deficits. Brain areas involved in biological motion perception, such STS (superior temporal sulcus) and MT (middle temporal) [25,36,37,49,52,53] are frequently mentioned as areas with abnormal structural and functional impairments in DS [13,15,54].
Thus it may be the case that the impaired processing of biological motion, such as observed in the current study and previous studies [29,35] may arise independently from motor impairments. Future research should focus on investigating these two possibilities. It may be the case that individuals with DS are unable to discriminate between gross motor movements as well discriminate differences of movement velocities, or attribute functionality to particular movements [29,35]. In addition it may be that an important attribute of motor dysfunction in DS individuals pertains to the inability to quickly combine visual and motor inputs [30,55]. Our results build on these previous findings, as we show that the discrimination of biological motion portraying the same actions produced at different speeds is also impaired in Down syndrome. The results of the control experiment suggest that DS individuals are not impaired on a more generalized motion task, suggesting that this cannot account for biological motion processing deficits see also [35].
Previous research has shown that individuals with DS are able to perceive biological motion stimuli [29,35], however their performance is impaired compared to healthy control subjects. Thus, although biological motion processing is impaired in DS it is evidently not completely absent. To ensure that subjects in the current study could perceive biological motion, before beginning the experiment they were shown a point light walker and were asked to identify what they see. Subjects who did not spontaneously report seeing a person were excluded from the study. Eight subjects were excluded on these grounds as mentioned at Procedure section above. As such, we would argue that the impaired performance reported here is not related to a more generalized inability to perceive biological motion. Nonetheless, in the current study performance was at chance level for subjects with Down syndrome, implying that they were unable to perform fine discriminations between the walking speeds of point light stimuli. In comparison, control subjects performed the task well, suggesting that there is a severe deficit in the ability to judge the speed of PLW gait patterns.
Deficiencies in biological motion perception could potentially impact on social functioning, as the recognition of social cues often requires the recognition of, and discriminations between actions. However, problems with social functioning are not commonly reported in individuals with DS [56]. Our experimental results suggest that individuals with DS cannot discriminate fine cues and thus they may use other strategies for social perception and interaction. The strategies, visual or otherwise, people with DS use to interact socially, however, are unclear from the current study. The inability to perform fine motor discriminations however may have additional impacts besides social functioning, as visually comparing walking speeds is a usual day-today task for humans and is required for a number of activities, such as avoiding other people on the sidewalk and playing team sports.
The purpose of the current study was to further develop the understanding of how individuals with DS process visuomotor information, specifically the motions produced by other people performing actions in different speeds. While we argue that the results of our study may be produced by interactions between cortical networks governing visuomotor process, the study is somewhat limited by the fact that we could only assess the outcome behaviorally. We did not measure the any brain activity of the participants, which could potentially offer further insights into how visual information, particularly that produced by other moving people, is processed in DS. Another potential limitation could stem from the fact that we did not measure eye-movements. To limit the role of extraneous eye moments an experimenter was present during testing, partially to ensure that subjects maintained fixation on the display. Walkers always appeared at the same location in the center of the screen and we suggest that it is unlikely that large eye movements significantly affected results in the current research. Furthermore, eye movements are not generally thought to play a major role in biological motion processing and PLWs can be processed accurately in the periphery [57,58]. A final limitation of the current study could be the range of walking speeds used. Ideally, a wider range of speeds would reveal the point at which biological motion perception improves for subjects with DS and drops to chance in the CG. This is problematic, however, as can be seen even for the current range of stimuli, differences between the CG and DS group were vast. A stimulus set that encompassed both chance level performance for CG subjects and adequate performance for the subjects with DS would thus have to be large and contain numerous stimuli that are either exceedingly difficult for subjects with DS or easy for control subjects. Instead we chose levels that adequately demonstrated the marked difference between these two groups.
In summary, the results of our study demonstrated that individuals with DS may have problems discriminating between biological movements presented at different speeds and also took longer RT to respond to PLW stimuli. These deficits can potentially be related to their motor learning impairments or a more generalized deficit in biological motion processing. Further research is required to elucidate how the visual motion produced by other people is processed by individuals with DS. This may have potential ramifications for how movements are taught to populations with DS. We suggest that teaching strategies that rely heavily on demonstrative body motion and actions may be ineffective for teaching individuals with DS. Verbal descriptions of movements do not require the perception of biological motion, and could potentially enhance the understanding of movements for individuals with DS. Additionally, our experimental results point towards potential hazards in the day-to-day lives of individuals with DS, for example when crossing streets, riding a bicycle, or catching objects in the air, in which the ability to compare locomotors speeds is necessary.
This work was supported by the National Council of Scientific and Technological Development, CNPq; Brazil and German Academic Exchange Service, DAAD, Germany, 290016/2014-2, 2017.
The studies in this manuscript were reported as part of a doctoral thesis by JWTC. Thanks are extended to the partners Institutions Papst Johannes Schule in Muenster, Germany and Associacao de Pais e Amigos da Crianca Deficiente in Volta Redonda, Brazil. Thanks are also extended to Dr. Sentot Santoso and Max Reith for preparing and improvement of the final manuscript and data acquisition, respectively.