
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2023)Volume 13, Issue 4
Introduction: As first products of Hemophilia gene therapy were recently approved by the European Commission (EC) and Food and Drug Administration (FDA) understanding patient’s fears, expectations and acquaintance of data is of high interest. Our aim was to investigate the knowledge and concerns of our patients and their caregivers regarding gene therapy and what are the misconceptions that exist.
Methods: A questionnaire including 18 questions assessing demography, hemophilia history/treatment, knowledge, fears and expectations regarding gene therapy treatment for hemophilia were collected from 100 patients with severe hemophilia and their caregivers.
Results: One hundred questionnaires were completed by 65 patients and 35 parents (primary caregivers of patients) with a median age of 36 years (18-75 years). Five of the respondents have previously received a gene therapy product for hemophilia and thus they answered a modified questionnaire. The greatest concern of the participants was that there is not enough data regarding complications and possible liver damage. Only 50/95 knew that Adeno- Associated Viruses (AAV) modified gene therapy will affect only the patient undergoing this treatment. Surprisingly 21/95 think that the mutation will be corrected and therefore hemophilia will not be transferred to their off-springs following gene therapy. Interestingly, among patients with Hemophilia A, the subgroup of Eemicizumab treated patients had more knowledge and more realistic expectations.
Discussion and conclusion: While most of the patients have information about gene therapy, their understanding is limited. In conclusion, this study highlights the need to work with patients, families and pharma companies in order to create better educational resources regarding gene therapy.
Hemophilia; Gene therapy; Adeno Associated Virus (AAV); Fear; Clinical trial
The field of hemophilia treatment has made enormous progress during the last decade. Novel therapies have been registered and multiple ones are in clinical studies [1,2]. The paradigm of replacement therapy has been challenged by the new medications that target other aspects in the coagulation cascade.
Extensive progress in gene therapy has been achieved recently in studies on Adeno-Associated Virus (AAV) vector with a B-domaindeleted FVIII and AAV vector with the Padua variant of FIX [3-5]. They demonstrated improvement in endogenous factor levels over sustained periods, significant reduction in annualized bleeding rates and lower exogenous factor usage.
However, new treatment modalities raise concern in patients. Lack of knowledge and misinformation create anxiety and prevent patients’ willingness to change their current therapy.
Gene therapy has the potential to revolutionize treatment for patients with hemophilia and recently the first products were approved by the European Commission (EC) and Food and Drug Administration (FDA) [6,7]. Patients and public perceptions could play a vital role in the development of therapies and influence their subsequent uptake [8]. Patients and caregivers need to be provided with adequate and accurate information which may influence their perception and acceptance of gene therapy.
Given the expanding evidence both physicians and patients will need sources of clear and reliable information to be able to discuss and judge the risks and benefits of treatment and provide informed consent about participation in clinical trials and licensed routine administration [8,9].
Understanding patients’ fears and expectations as well as knowledge gaps will be valuable in order to implement better education and alleviate concerns. Working with patient partners in the co-design of what educational resources are needed, is crucial to addressing the issues. Khair et al. previously investigated patients’ fears of gene therapy in a small cohort [10,11].
Our centre is a tertiary large Hemophilia Center that follows and treats over 700 patients with hemophilia. The centre is actively involved in clinical trials of gene therapy for severe hemophilia patients since 2019. Our aim is to investigate the knowledge and concerns of our patients and their parents regarding gene therapy and what are the misconceptions that exist.
We performed a cohort study among our adult hemophilia patients. Parents of hemophiliac children were also included. The study was approved by the local Institutional Review Boards (IRB) and all participants/parents signed an informed consent.
Any severe adult hemophilia patient or caregiver (parent) coming for a routine visit at the centre during the study period (May 2021-March 2022) was offered the option to answer a specific questionnaire addressing patients’ knowledge and attitudes towards gene therapy following consent. The questionnaires were answered anonymously and the data was collected into an excel sheet for statistical analysis.
The Questionnaire was formed by two Hematologists specializing in the treatment of hemophilia patients, in our centre. The questionnaires included 18 questions addressing patients’ demography, hemophilia history/treatment, knowledge, fears and expectations regarding gene therapy treatment for Hemophilia A different questionnaire was provided for patients that have received a gene therapy product. (Shown in supplements 1 and 2 respectively).
Statistical analysis
Statistical analysis was performed with International Business Machines Statistical Package for the Social Sciences (IBM SPSS) Statistics (version 23.0; IBM Corp). Continuous variables were presented as median (Interquartile Range (IQR) or range, as indicated). Categorical variables were presented as counts, proportions and/or percentages. The Mann-Whitney U test was used to compare two patient subgroups for continuous variables. The Fisher’s exact test of Chi-square test were used to compare two patient subgroups for categorical variables. Two-tailed P values of less than 0.05 were considered statistically significant.
Patients and demographics
One hundred questionnaires were completed by 65 patients and 35 parents of paediatric patients. Five of the adult patients that were treated by a gene therapy clinical trial completed another (different) specific questionnaire. The median age of the responders was 36 years (18-75 years). Thirty-eight out of the total group had previous FVIII/FIX inhibitor or a family history of inhibitor. Demographic data and hemophilia history of our participants are summarized in Table 1.
| Total | Hemophilia Patients | Hemophilia Patients’ parents | Patients that went through gene therapy | ||
|---|---|---|---|---|---|
| N | 100 | 60 | Mothers | Fathers | 5 |
| 20 | 15 | ||||
| Median Age (years) | 36 | 36 | 37 | 29 | |
| Median age of sons with hemophilia (years) | - | - | 6 | - | |
| - | |||||
| Median Education (years) | 15 | 14 | 15 | 15 | |
| Employed (%) | 76.6 | 75 | 79.4 | 100 | |
| Hemophilia A (n) | 78 | 46 | 28 | 4 | |
| Hemophilia B (n) | 22 | 14 | 7 | 1 | |
| Previous/current FVIII/FIX inhibitor (n) | 25 | 8 | 17 | n/a | |
| Family history of FVIII/FIX inhibitor (n) | 13 | 8 | 5 | n/a | |
Table 1: Demographic data of our cohort.
The current Hemophilia treatment of the study group is presented in Figure 1. Forty percent of the patients included in the study were on Emicizumab treatment. Interestingly as can be seen in Figure 2 the Patients treated with Emicizumab were more educated than the other adult patients (median, 16 years of education vs. 12 years, P=0.036).
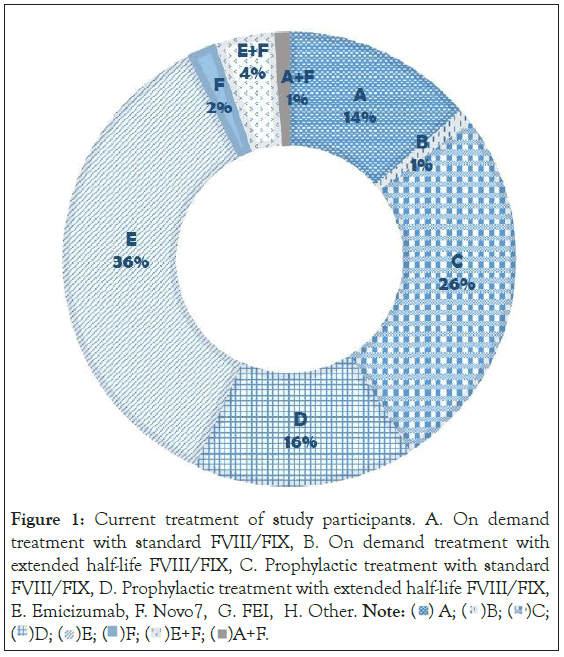
Figure 1: Current treatment of study participants. A. On demand
treatment with standard FVIII/FIX, B. On demand treatment with
extended half-life FVIII/FIX, C. Prophylactic treatment with standard
FVIII/FIX, D. Prophylactic treatment with extended half-life FVIII/FIX,
E. Emicizumab, F. Novo7, G. FEI, H. Other. Note: 
 A+F
A+F
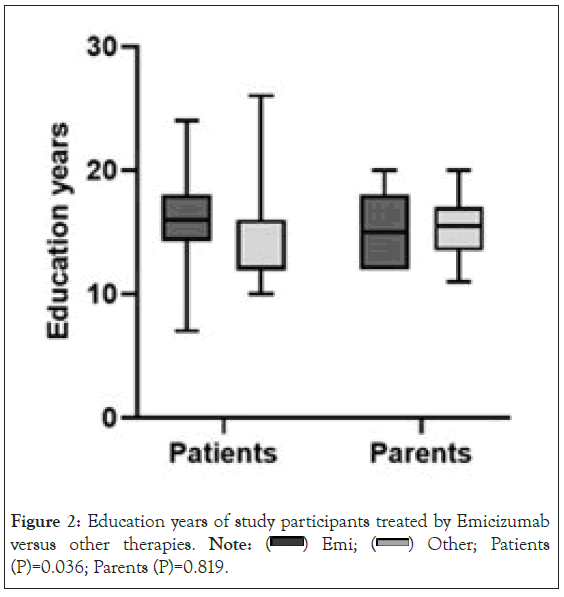
Figure 2: Education years of study participants treated by Emicizumab
versus other therapies. Note:  Other; Patients (P)=0.036; Parents (P)=0.819.
Other; Patients (P)=0.036; Parents (P)=0.819.
Knowledge and perceptions regarding gene therapy for hemophilia: The 82% of responders have previously heard about gene therapy for hemophilia. The majority of the responders (74.4%) heard about gene therapy for hemophilia at the hemophilia treatment centre, 48.7% through the hemophilia association and 20.5% from either international congresses or publications.
More than 80% of the study participants would like to get more information about gene therapy. It was noted that the participants treated with Emicizumab, including both adult patients and parents of pediatric patients were slightly more interested to learn more about gene therapy than the other participants (not statistically significant, 92.1% vs. 80.7%, P=0.145).
The 90% of patients and 86% of parents were willing to consider gene therapy in the future if they receive more data (Figure 3).
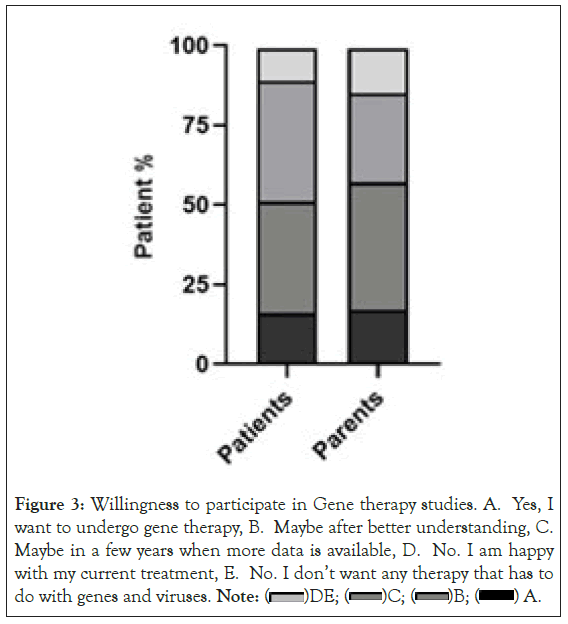
Figure 3: Willingness to participate in Gene therapy studies. A. Yes, I
want to undergo gene therapy, B. Maybe after better understanding, C.
Maybe in a few years when more data is available, D. No. I am happy
with my current treatment, E. No. I don’t want any therapy that has to do with genes and viruses. Note:  A.
A.
Patients with previous/current inhibitor to FVIII and non-inhibitor patients were similarly interested in gene therapy (80% vs. 91.4%, respectively, P=0.468).
Only 50/95 knew that AAV modified gene therapy will affect only the patient undergoing this treatment. Surprisingly 21/95 thought that the mutation will be corrected and therefore hemophilia will not be transferred to their offspring following gene therapy.
Sub analysis of respondents of 18-40 years of age (35 patients) revealed that 6/35 never heard of gene therapy. However only 2/35 stated that they are not interested to learn about or receive this kind of treatment in the future. Concerns and expectations regarding gene therapy for hemophilia.
The greatest concern of the participants was that there is not enough data regarding complications and possible liver damage. The main fear of the patients’ group that underwent gene therapy was that the therapy will not be maintained over the years and there will be no option to repeat the therapy.
Interestingly, when analysing the expectations from gene therapy the main expectation in the Emicizumab group (n=38) was "No severe hemophilia for 5-10 years" (20/38). In the rest of the respondents the most common expectation was “No need for additional replacement therapy ever” (38/57) (Figure 4).
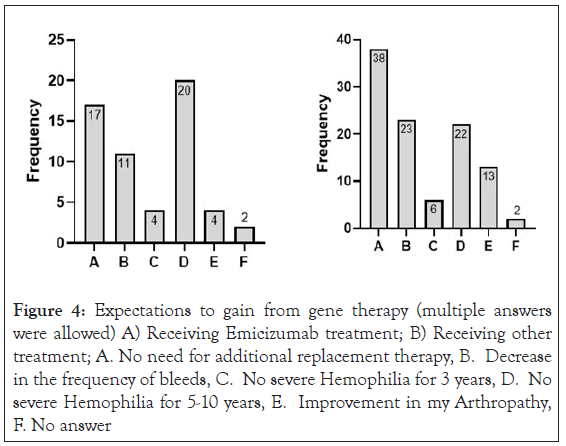
Figure 4: Expectations to gain from gene therapy (multiple answers were allowed) A) Receiving Emicizumab treatment; B) Receiving other treatment; A. No need for additional replacement therapy, B. Decrease in the frequency of bleeds, C. No severe Hemophilia for 3 years, D. No severe Hemophilia for 5-10 years, E. Improvement in my Arthropathy, F. No answer
When investigating if the availability of subcutaneous non replacement therapy options changes the decision regarding receiving gene therapy, 41/95 answered “Yes”.
Out of 91 respondents to the question whether the option of gene therapy would change thoughts regarding possible pregnancy outcomes (with suspected hemophilia of the embryo) 36 answered “Yes”, these comprised of 45.7% from the Emicizumab treatment group and 35.7% from the other treatment group (P=0.343).
Genetic therapies are now a therapeutic reality for some diseases with at least 27 cell therapy and gene therapy products approved by the FDA for treating inherited diseases and several types of cancer [12]. Recently the first gene therapy products for hemophilia were approved by the EC and FDA [6-7]. This study is the largest study examining perceptions regarding gene therapy among hemophilia patients and their caregivers in a large tertiary centre with potential access to novel therapies. Our national hemophilia centre, treating over 700 patients, has recently been involved in several companysponsored gene therapy clinical trials. Gene therapy products’ registration would be offered soon to patients with hemophilia, thus we wanted to explore patients’ knowledge and thoughts regarding the process.
The patients that responded to our questionnaire were mostly young (median age below 40 years), well taken care of (receiving prophylaxis therapy), educated (at least high school education) and working. While four patients were older than 70 years, we understand that their answers are probably non-representative for elderly hemophilia patients with comorbidities.
A previous study of Khair et al., investigated parental prospective on gene therapy for children [10]. This study included mostly mothers (60/63) of hemophilia (not all patients had severe hemophilia). They concluded that information and news about gene therapy should be provided to families living with hemophilia. In another study from the same group including 10 men with severe hemophilia it was shown that there are many concerns about gene therapy including safety, efficacy. Their study highlighted patients' concern that factor levels may decline with time resulting in a need to return to prophylaxis therapy [11]. Similarly, our results raise patients’ concerns regarding safety and long term effects of gene therapy. Nonetheless our cohort was significantly larger consisting of both patients and parents (including fathers as well as mothers). A large percentage of the patients are currently treated by nonreplacement therapy (Emicizumab prophylaxis).
As our centre currently treats over 150 patients with Emicizumab prophylaxis, analysing this hemophilia sub-group was of high interest. The Emicizumab treated patients were slightly more educated than the rest of the group raising the possibility that more educated people are more open to new therapies such as gene therapy. The Emicizumab patients’ expectations were also more realistic as compared to the rest of the group, they anticipate gene therapy effect to last for several years rather than becoming a lifelong cure.
Notably, most of the patients have information about gene therapy (mainly derived from the hemophilia Center), however their understanding is limited as some didn’t fully understand the mechanism and didn’t realize that the treatment will influence only themselves and not prevent hemophilia inheritance to their future daughters.
In addition, among the subgroup of the 18-40 years old, a target population for gene therapy, a substantial percentage never heard of gene therapy, which emphasizes the need for better patient education. Despite the fact that the majority of gene therapy studies exclude inhibitor patients to date, these patients were interested as much as non-inhibitor patients in receiving gene therapy.
Peyvandi et al., conducted a survey among 201 health care practitioners and scientists and reviled that the knowledge gap exists not only in patients, as highlighted in our study, but in health care providers as well [13].
Their survey lead to the development of "Gene therapy in hemophilia: An ISTH education initiative".
Our study supports the notion that there is still lack of knowledge of patients regarding gene therapy. As this mode of therapy is becoming realistic and not only in clinical trials there is a need for better patient education.
In conclusion, this study highlights the need to work with patients and families to create educational resources regarding gene therapy.
Research grant from Pfizer.
Data was collected from questionnaires completed by patients following informed consent.
The study was approved by the Institutional Review Board (IRB) of Sheba Medical Center in accordance with the declaration of Helsinki and the patients or caregivers that completed the questionnaire provided informed consent.
The authors have no competing interests, except from Prof. G. Kenet:
Grant/Research support/funding - BSF, Opko Biologics, Pfizer, Roche, Shire
Consultation - ASC therapeutics, Bayer, Biomarine, Novonordisk, Pfizer, Roche, Sanofi- Genzyme,Sobi, Takeda, Uniquore
Honoraria-Bayer, BioMarin, BPL, CSL, Pfizer, Novonordisk, Roche, Sanofi-Genzyme, Sobi, Spark, Takeda, Uniquore.
Dr. AA Barg: Honoraria from ROCHE
Dr. S. Levy-Mendelovich: Grants - Pfizer, NovoNordisk. Honoraria - Pfizer
We thank Orly Katz for her contribution in editing the paper.
SLM and TBB wrote the questionnaire, collected from patients, wrote the manuscript draft, critical review of the manuscript, collected and interpreted the data.
IB did statistical analysis and reviewed the manuscript draft. MK and DB helped with data collection and reviewed the final draft.
AAB and GK wrote the manuscript draft and critically reviewed the manuscript.
In addition, all authors provided their final approval of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Barazani BT, Barg AA, Bashari D, Danin-Kreiselman M, Kenet G, Levy-Mendelovich S (2023) Perceptions and Fears of Hemophilia Patients and Caregivers Regarding Participation in Gene Therapy Clinical Trials. J Clin Trials. 13:534.
Received: 14-May-2023, Manuscript No. JCTR-23-24116; Editor assigned: 16-May-2023, Pre QC No. JCTR-23-24116(PQ); Reviewed: 30-May-2023, QC No. JCTR-23-24116; Revised: 06-Jun-2023, Manuscript No. JCTR-23-24116(R); Published: 13-Jun-2023 , DOI: 10.35248/2167-0870.23.13.534
Copyright: © 2023 Barazani BT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.