
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2022)Volume 12, Issue 1
Arsenic poisoning has been implicated in the illnesses and deaths of prominent historical figures such as Napoleon Bonaparte and King George III of Great Britain. Most recently, arsenic has gained media attention owing to public exposures through ground contamination of drinking water. The difficulties of recognizing the subtle and variable signs and symptoms of arsenic toxicity remain the main impediment to a timely diagnosis for a condition with limited treatment options. In this case, we will discuss a 51-year-old Caucasian male who suffered three hospitalizations spanning from October 2020 to August 2021 before the unifying diagnosis of arsenic toxicity was discovered.
Oxidative stress; Lipid peroxidation; VOCs; Antioxidants
Most clinicians in the United States will work their entire career without encountering a confirmed case of arsenic toxicity. The non-specific signs and symptoms of arsenic toxicity in the context of a low disease incidence, for our patient, exacerbated factors in our healthcare system that commonly lead to diagnostic error (i.e., cognitive bias and poor communication amongst treating physicians). In this case, our patient suffering from suspected acute on chronic arsenic toxicity underwent redundant testing and treatment for his symptoms of chronic, recurrent diarrhea and subsequent hypokalemia with muscular weakness. The diagnostic work-up in each of his hospitalizations was pursued with incomplete knowledge of or regard for prior testing. Eventually, the patient’s condition deteriorated to the point that a sufficiently broad work-up was pursued and the diagnosis of acute arsenic toxicity was finally confirmed. This case presents the clinical signs and symptoms and outcome of arsenic toxicity in our patient.
A 51-year-old male with a past medical history significant for Gastroesophageal Reflux Disorder (GERD) and recurrent hypokalemia presented to the hospital. In the Emergency Department (ED), vital signs were as follows: Temperature (T) of 36.9°C, Heart Rate (HR) 109 Beats Per Minute (BPM), Blood Pressure (BP) of 125/84 mmHg, mean arterial pressure (MAP) 97 mmHg, respiratory rate (RR) 17 breath/minute (b/m), and oxygen saturation of 97% on room air. Initial Complete Blood Count (CBC) was significant for White Blood Cell Count (WBC) 3.1, hemoglobin 9 grams per deciliter (g/dL), hematocrit 26.1%, and platelet count of 243,000 cells per microliter of blood (c/μL). Prothrombin time and international normalized ratio were 12.9 seconds and 1.16, respectively. D-dimer was 797 nanograms per milliliter (ng/mL). Initial Comprehensive Metabolic Panel (CMP) was significant for potassium 2.7 millimole per liter (mmol/l), total bilirubin 1.7 milligram per deciliter (mg/dl), Urine Drug Test (UDS) was negative, Thyroid Stimulating Hormone (TSH) was within normal range, Creactive protein 1.7 mg/dl, Erythrocyte Sedimentation Rate (ESR) 42 millimeters per hour (mm/hr), and lipase 581 unit per liter (U/L). Stool pathogen including Clostridium difficile was negative. Imaging including Computed Tomography (CT) of the head without contrast, chest x-ray, and CT scan of the abdomen and pelvis with contrast were normal.
Work-up during previous hospitalizations did not identify an etiology for his symptoms and no complicating features were observed. The patient reported temporary symptomatic improvement following an empiric fourteen-day course of antibiotics with ciprofloxacin and metronidazole. During the months that followed, he suffered recurrent episodic diarrhea associated with generalized muscle weakness. The apparent chronic, progressive, diarrheal illness prompted additional investigation that included a liver ultrasound and Hepatobiliary Iminodiacetic Acid (HIDA) scan; both of which were inconclusive for a cause.
His physical examination was significant for proximal muscle weakness in the lower extremities, diminished coordination, and myoclonus of bilateral lower extremities. Overnight on the day of his admission, the patient became encephalopathic and developed respiratory distress necessitating transfer to the Intensive Care Unit (ICU). A complete toxic evaluation was included in the work-up, including repeat UDS, as well as a Lumbar Puncture (LP) on suspicion of a progressive neurological syndrome and all were not significant. After several days, the heavy metal screen revealed a high blood arsenic level, followed by confirmatory 24-hour urine arsenic concentration that also exceeded 1000 micrograms per liters (mcg/l) which is the reference range. Toxicology was consulted and chelating therapy with dimercaptosuccinic acid was initiated starting at 10 mg per kilogram (kg) three times daily for five days, followed by 10 mg per kg for a total of fourteen days.
Unfortunately, the patient continued to experience gradual loss of strength in his bilateral upper extremities, eventually developing a “claw-hand” deformity as seen in Figure 1.
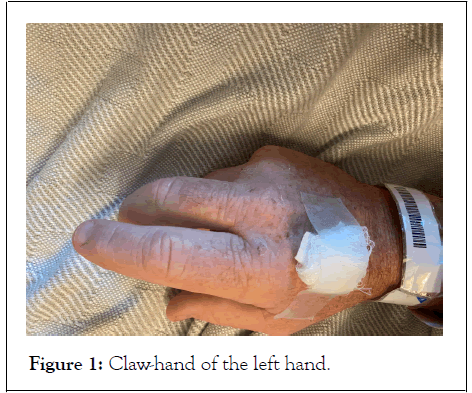
Figure 1: Claw-hand of the left hand.
Additionally, the patient suffered a small ischemic stroke of the left frontal lobe during his hospitalization. Patient was discharged after twenty-seven days of hospitalization and was sent to an inpatient rehabilitation facility where he experienced improvement in his upper extremity weakness.
On suspicion of a progressive neurological syndrome, and after ruling out other possible causes of neurologic deterioration as mentioned above, a complete toxic evaluation was performed in which the heavy metal screen revealed an undetectably high blood arsenic level. This was followed by a confirmatory 24-hour urine arsenic concentration that also exceeded the reference range [1-3].
As seen in previously reported case reports, acute encephalopathy with delirium, coma and seizures can develop and progress over several days after exposure to arsenic [4]. There have also been reports of more persistent central nervous system symptoms such as headache, confusion, and memory problems [4,5].
It has been suggested that axonal degeneration and segmental demyelination might be equally prominent pathological features of the neuropathy, depending on the dosage and the length of time of exposure to arsenic [6]. Most of the adverse effects of arsenic are caused by inactivated enzymes in the cellular energy pathway, whereby arsenic reacts with the thiol groups of proteins and enzymes and inhibits their catalytic activity. Furthermore, arsenic-induced neurotoxicity, like many other neurodegenerative diseases, causes changes in cytoskeletal protein composition and hyperphosphorylation. These changes may lead to disorganization of the cytoskeletal framework, which is a potential mechanism of arsenic-induced neurotoxicity [7]. Hyperkeratosis and scaling particularly diffusely on the palms and soles as seen in this patient, also are quite characteristic of arsenic poisoning (Figure 2).
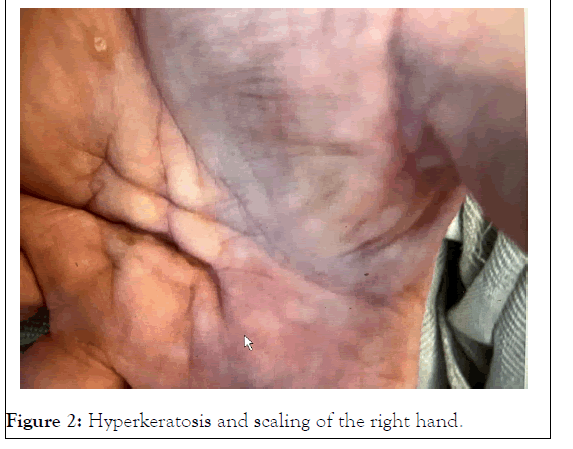
Figure 2: Hyperkeratosis and scaling of the right hand.
Measuring the urinary concentration of arsenic is useful in assessing recent exposure, and high-quality reference ranges are available for urinary arsenic concentrations in the U.S. population. Biomonitoring for arsenic in hair and nails has been used in many studies and is particularly useful in evaluating chronic exposures. Interpreting the health implications of arsenic concentrations in biological samples is limited by the small number of studies that provide information on the correlation and dose-response relationship between biomonitoring test results and adverse health effects [4].
In this case, chelation therapy with dimercaptosuccinic acid (succimer) was initiated after consultation with toxicology. Succimer is an analog of dimercaprol, which forms water soluble chelates with heavy metals such as arsenic which are subsequently excreted renally. Succimer binds heavy metals; however, the chemical form of these chelates is unknown. Spot arsenic levels were monitored after initiation of chelation agent and were shown to gradually decrease levels; However, 24-hour urine arsenic urine concentration continued to exceed 1000 mcg/L.
This case highlights the importance of carefully considering a thorough differential diagnosis, guarding against cognitive bias, and striving to promote better communication and interoperability in healthcare. The obvious rarity of arsenic toxicity presents a diagnostic dilemma made worse by its nonspecific symptoms that can often be attributed to other underlying health condition. Review of literature and historic accounts confirm that patients with arsenic toxicity do not present with discrete symptoms that can be readily linked to exposure. Unfortunately, for victims of this malady, early identification, and elimination of the source of exposure is paramount for their prognosis.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sakaan R, Stewart C, Knight K, Long B (2022) Perfect Poisoning: Arsenic Induced Neurotoxicity in a Relatively Healthy Patient. J Clin Toxicol. 12:500.
Received: 04-Jan-2022, Manuscript No. JCT-22-15755; Editor assigned: 07-Jan-2022, Pre QC No. JCT-22-15755 (PQ); Reviewed: 18-Jan-2022, QC No. JCT-22-15755; Revised: 24-Jan-2022, Manuscript No. JCT-22-15755 (R); Published: 31-Jan-2022 , DOI: 10.35248/2161-0495-22.12.500
Copyright: © 2022 Sakaan R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.