
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Case Report - (2020)Volume 11, Issue 9
Background: Kartagener syndrome (KS) is a partial disease of primary ciliary dyskinesia with defects in the fine structure of cilia and flagella. KS is an autosomal recessive inherited disease with three major features: visceral inversion, chronic sinusitis, and bronchiectasis. Lung transplantation is an option for end-stage KS, which results in respiratory insufficiency. However, there have been few reports on perioperative management of lung transplantation in patients with KS.
Case presentation: A 44-year-old female who was diagnosed with KS in infancy had tracheotomy at age 42 to prevent infection, and was placed on standby for lung transplantation under a ventilator. After a two-year wait, she underwent bilateral brain-dead lung transplantation. In parallel with induction of anesthesia, the left femoral artery and vein were taped to allow extracorporeal circulation in case of a sudden change, and extracorporeal circulation was started after opening the chest, as planned. As a general anesthetic, propofol, which has a lower inhibitory effect on airway ciliary movement, was mainly used. After both pneumonectomies were performed, the upper airway was washed. A donor left whole lung was then transplanted into the left chest cavity, and a donor right lung with resection of the middle lobe was transplanted into the right chest cavity. Pulmonary physiotherapy and bronchoscopic expectoration were encouraged, and the patient was weaned from the ventilator on postoperative day (POD) 13 and discharged from hospital without oxygen on POD 101.
Conclusion: Perioperative respiratory infection control is of paramount importance in bilateral lung transplantation for KS, and a good clinical course was achieved through careful infection control.
Kartagener syndrome; Lung transplantation; Anesthesia; Primary ciliary dyskinesia
KS: Kartagener Syndrome; POD: Postoperative Day; NPPV: Noninvasive Positive Pressure Ventilation; VC: Vital Capacity; RV: Residual Volume; TLC: Total Lung Capacity; RBCs: Red Blood Cells; FFP: Fresh Frozen Plasma; PC: Platelet Concentrate; MRSA: Methicillin-resistant Staphylococcus aureus.
Kartagener syndrome (KS) is an autosomal recessive inherited syndrome with visceral inversion, chronic sinusitis, and chronic bronchiectasis as its three main characteristics [1]. KS accounts for approximately 50% of cases of primary ciliary dyskinesia, which is a motility disorder caused by micro-structural abnormalities of the striae and flagella [2]. An early fetal striae movement abnormality results in visceral inversion. In addition, decreased clearance of sputum discharge due to impaired airway striae movement and abnormalities in neutrophil migration lead to repeated airway infections and bronchiectasis in KS [3,4].
In Japan, where cystic fibrosis is less common, lung transplantation for infectious lung disease is rare, especially for KS. We report here a bilateral brain-dead lung transplantation procedure in a patient with end-stage KS who had repeated infections and severe respiratory failure for a long period of time.The lungs are in direct contact with outside air after transplantation, and for this reason, cases of lung transplantation are more likely to develop infections, compared to other organ transplantations. Thus, especially in lung transplantation for infectious lung diseases, including KS, control of infection is often the key to success [2,3].
The patient was a 44-year-old female of height 162 cm and weight 46 kg. After diagnosis of KS in childhood, respiratory decline gradually developed. At 39 years of age she was diagnosed with pulmonary hypertension and type II respiratory failure, and was started on pulmonary vasodilators, home oxygen therapy and noninvasive positive pressure ventilation (NPPV) management. At age 42, due to exacerbation of pneumonia, tracheotomy was performed to separate the upper and lower airways to prevent infection. She was then placed on a waiting list for lung transplantation under a ventilator. After a two-year wait, she underwent brain-dead bilateral lung transplantation.
Preoperative pulmonary function tests showed %VC (vital capacity) 36.6%, VC 1.18L, %RV (residual volume) 133.3%, RV 1.64 L, %TLC (total lung capacity) 64.0%, and RV/TLC 57.5%, with severe impaired restrictive ventilation and increased residual air volume and residual air rate (Table 1). Sputum culture tests revealed Pneumococcus pneumoniae and Pseudomonas aeruginosa. Imaging studies showed visceral inversion, marked bronchiectasis and lobar-centric granular to nodular shadows, a so-called tree in bud appearance, in the lung field (Figures 1, 2a and 2b). Immediately before surgery, a low dose of clarithromycin was given as an antibiotic, and the endothelin receptor antagonist, bosentan hydrate, and PDE-5 inhibitor, sildenafil, were administered as treatment for pulmonary hypertension.
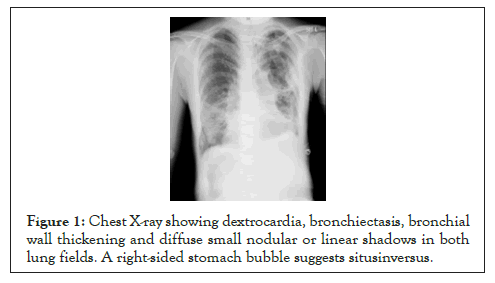
Figure 1: Chest X-ray showing dextrocardia, bronchiectasis, bronchial wall thickening and diffuse small nodular or linear shadows in both lung fields. A right-sided stomach bubble suggests situsinversus.
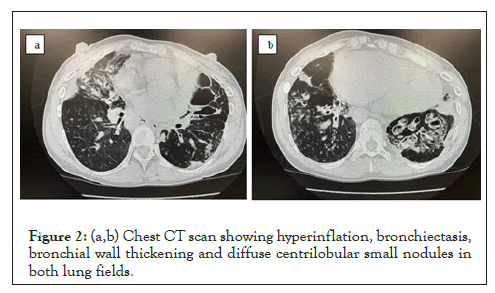
Figure 2: (a,b) Chest CT scan showing hyperinflation, bronchiectasis, bronchial wall thickening and diffuse centrilobular small nodules in both lung fields.
| Hematology | Blood chemistry | Pulmonary function test | ||||
|---|---|---|---|---|---|---|
| Ht | 36.80% | TP | 7.4 g/dl | VC | 1,180 ml | |
| Hb | 11.7 g/dl | Alb | 4.1 g/dl | %VC | 36.60% | |
| RBC | 4.07 × 106/µl | GOT | 19 U/l | FEV1.0 | 1,170 ml | |
| WBC | 15,000/µl | GPT | 14 U/l | FEV1.0%-G | 98.30% | |
| Plat | 29.1 × 104/µl | LDH | 169 U/l | RV | 1,640 ml | |
| PT | 19.5 sec | T-bil | 1.7 mg/dl | %RV | 133.30% | |
| PT (INR) | 0.96 | Amy | 22 U/l | TLC | 2.850 ml | |
| APTT | 29.8 sec | BUN | 12 mg/dl | %TLC | 64.00% | |
| D-dimer | 0.6 µg/ml | Cr | 0.40 mg/dl | RV/TLC | 57.50% | |
| CRP | 0.20 mg/dl | DLco | 9.07 ml/min/mmHg | |||
| Arterial blood gases | BNP | 5.7 mg/dl | %DLco | 39.30% | ||
| FiO2 | 0.6 (SIMV+PS) | Na | 139 mEq/l | - | - | |
| pH | 7.301 | Cl | 105 mEq/l | - | - | |
| PO2 | 72.6 mmHg | K | 4.6 mEq/l | - | - | |
| PCO2 | 88.5 mmHg | - | - | - | - | |
| BE | 12.7 mmol/l | - | - | - | - | |
| HCO3- | 42.7 mmol/l | - | - | - | - | |
Table 1: Laboratory data.
Due to the extremely poor respiratory status, the presence of granulation around the tracheal incision (Figure 3), and the expectation of difficulty in differential lung ventilation with a double-lumen tracheal tube because of visceral inversion, we decided not to perform differential lung ventilation by replacing the tracheal incision cannula, but rather to conduct this procedure under cardiopulmonary bypass from the beginning of surgery. The patient was admitted to the operating room in a wheelchair and was relatively well oxygenated under a ventilator, but had marked hypercapnia (PaCO2 88.5 mmHg) (Table 1). In parallel with induction of anesthesia, the left femoral artery and vein were taped so that extracorporeal circulation could be used in case of a sudden change. However, as planned, after opening the chest, we started cardiopulmonary bypass with the left femoral vein, superior vena cava devascularization, and ascending aortic transmission. Anesthesia was maintained with propofol as a general anesthetic, since it is less inhibitory for airway striae movement compared to volatile anesthetics [5].
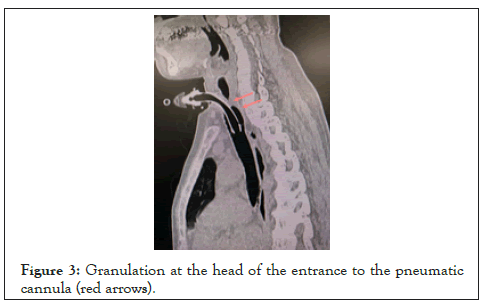
Figure 3: Granulation at the head of the entrance to the pneumatic cannula (red arrows).
After removal of both lungs, each thoracic cavity was spread with 60 mg of tobramycin and washed with 10 L of warm saline solution. After a bronchoscopic upper airway flush was performed by anesthesiologists, the donor left whole lung was transplanted into the left thoracic cavity, and the donor right lung with the middle lobe removed was transplanted into the right thoracic cavity. In KS, due to visceral inversion, the running and length of the main stem bronchus are reversed, but this did not cause any technical problems that may have occurred in tracheal anastomosis. A pulmonary artery transection was located caudal to the recipient left bronchus, and the donor left bronchus was anastomosed to the lower lobe bronchus and the upper lobe bronchial transection was closed.All aspirated blood during cardiopulmonary bypass was discarded due to concerns about infection. Weaning from cardiopulmonary bypass was relatively smooth.
The operation time was 9 h 24 min, anesthesia time was 11 h 18 min, and cardiopulmonary bypass time was 4 h 20 min. Intraoperative hemorrhage was 8825 ml and the patient was transfused with 1960 ml of red blood cells (RBCs), 2860 ml of fresh frozen plasma (FFP), and 800 ml of platelet concentrate (PC). Because the vagus nerve is blocked in the transplanted lung, sputum tends to accumulate in the lung. For this reason, postoperatively, the patient was repositioned every 2 h, and pulmonary physiotherapy and bronchoscopic expectoration were encouraged. Sputum collected by bronchoscopy was submitted daily for culture studies and used as an indicator for the antibiotics used. The patient was managed in a separate room in the ICU and the number of contacts was limited.She was discharged from the ICU on postoperative day (POD) 9, weaned from the ventilator on POD 13, and discharged from hospital on POD 101 with no oxygen needed.
The most important task in postoperative management of lung transplantation, in which the frequency of infections is higher than in other organ transplantations, is control of rejection and infection. The lung is made up of as many as 50 different types of cells and is more likely to be rejected than other organs, which requires administration of many immunosuppressive drugs. Furthermore, in transplants from brain-dead donors, potential infections may be present due to aspiration and ventilatory management. Therefore, infection control is extremely important, especially in lung transplantation for infectious lung diseases, including KS [2-4].
In Japan, where cystic fibrosis is less common, lung transplantation for infectious lung disease is rare and there are few reports of lung transplantation for KS, in particular. Because of the complication of sinusitis in KS, if the period of time when the patient is on a ventilator is prolonged before surgery, as in this case, an aggressive tracheostomy is performed to separate the upper and lower airways. At the time of lung transplantation, the vagus nerve is detached and the cough reflex of the peripheral airways is lost. Therefore, postoperatively, we use position changes, pulmonary physiotherapy, and frequent sputum manipulation.
The present case was compared with seven previous cases of bilateral lung transplantation for infectious lung disease (6 females, 1 male) performed at our hospital [6]. These cases included two each of primary ciliary dyskinesia, cystic fibrosis, and bronchiectasis of unknown origin, and one of diffuse panbronchiolitis. Two patients were on a ventilator with Hugh-Jones classification IV-V status requiring oxygenation for 24 h, and both had hypercapnia. All cases underwent preoperative positional drainage, nebulizer inhalation, and bronchoscopic toileting. Five of the seven patients had sinusitis, and three diagnosed with active sinusitis also had sinus drainage under local anesthesia or general anesthesia before lung transplantation. Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) were detected preoperatively, but fortunately were susceptible to antibiotics, which were administered several days before transplantation. All patients were treated with cardiopulmonary bypass, and no postoperative pneumonia occurred. After surgery, all patients had dramatic improvement to a Hugh-Jones classification of I. All were released from oxygen therapy and were able to return to society. Only one case of singlelung transplantation showed chronic rejection, but quality of life was still well maintained because this was localized to one side. From 5 months to 12 years after transplantation, all patients, including the present case, are alive.
Perioperative respiratory infection control is important in lung transplantation for patients with KS. Good intraoperative management can be achieved with thorough respiratory infection control, promotion of secretion, and confirmation of anatomical structures.
Written consent was obtained from the patient for publication of this case report
All data generated or analyzed in this study are included in this article.
The authors declare no conflicts of interest associated with this manuscript.
Institutional sources only.
SK wrote the initial draft of the manuscript. SK and AS performed perioperative management. SK was the attending anesthesiologist for the case. SK supervised perioperative management. CT, TT and KF drafted and edited the manuscript. All authors read and approved the final manuscript.
Not applicable.
Citation: Kawamoto S, Shiraki A, Takeda C, Tanaka T, Fukuda K (2020) Perioperative Management of Brain-Dead Bilateral Lung Transplantation in a Patient with Kartagener Syndrome: A Case Report. J Anesth Clin Res. 11: 968.
Received: 07-Sep-2020 Accepted: 21-Sep-2020 Published: 28-Sep-2020 , DOI: 10.35248/2155-6148.20.11.968
Copyright: © 2020 Kawamoto S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.