Journal of Theoretical & Computational Science
Open Access
ISSN: 2376-130X
ISSN: 2376-130X
Research Article - (2023)Volume 9, Issue 4
Anemia, being one of the most significant genetic haematological disorders afflicting people of Indian descent, has been managed with it as well as a plethora of other pathological conditions. Throughout this investigation, potential target proteins for drugs and bio-actives are identified and evaluated. Analyses of bioactive agent’s in silico anti-sickling activity were conducted. Chlorogenic acid and catechin, the bio-actives found in wheat grass and the betel plant, are being studied against two anemia related target receptors: Hepcidin and transferrin. The assessment was made using the binding-free energy value as well as the different interactions between the amino acids at the receptors and the ligand. Detailed evaluation of the ligands' binding positions revealed the appearance of desirable interactions, such as hydrogen bonds, π-cation, Van der waals, and hydrophobic bonds. The results strengthen the body of research supporting the use of natural bio-actives in the treatment of anemia.
Molecular docking; Hepcidin; Transferrin; Anemia; Chlorogenic acid; Catechin
One quarter of the global population suffers from anemia, a health issue [1]. It affects people of all ages and even from almost every country, nonetheless, it affects children and pregnant women more frequently [2]. As in the majority of third world nations, anemia has reached epidemic proportions [3]. According to statistics, the most common micronutrient insufficiency, iron deficiency, is just to account for 50% of anemia cases. According to the WHO, hemoglobin levels below 12 gram percent for women and below 13 gram percent for men are considered to be anemic [4,5]. Four subunits make up the globular protein known as Hemoglobin (Hb). Two of these subunits stand in for chains, while the other two stand out for chains [6]. A heme group with an iron atom in the middle makes up each subunit. The deoxyhemoglobin molecule (deoxyHb) is a form of Hb that contains no other additional molecules. Patients with iron deficiency anemia have high serum levels of the hepatic hormone hepcidin, which regulates iron metabolism (IDA) [7]. Numerous studies have shown that some anemic people may not benefit from taking oral iron supplements. These individuals' have high blood hepcidin levels which increase the restriction of iron absorption, leading to a persistent iron deficiency. To treat this condition, different methods are designed to block the formation of hepcidin. While antigen therapy aims to reduce hepcidin mRNA, the most frequently used treatments affect hepcidin synthesis through iron signaling, inflammatory, and erythropoiesis pathways.
The blood plasma protein known as human Transferrin (hTf) is widely known for its function in the transport of iron. This protein may be able to carry additional metal ions or organometallic compounds from the bloodstream to all cell tissues, even if it only has 30% of its Fe+3 binding capacity. A crucial part in preserving iron homeostasis is played by the therapeutically relevant protein known as human Transferrin (hTf) [8]. Studies on hTf are important because iron accumulation in the CNS plays a crucial role in neurodegenerative diseases. HTF is a glycoprotein, with a molecular mass of 79.6 kDa and 679 amino acid residues. Since they contain therapeutic capabilities, medicinal plants, known for their illustrious history, have been frequently used as medical treatments. Various investigations and clinical trials have tested with these substances' primary ingredients, which serve as replacements for various medicinal goals [9-11]. The creation of numerous drugs that have been used successfully for numerous disorders was made possible by medicinal plants. Triticum aestivum L., also referred as wheat grass, is a member of the poaceae or gramineae family of monocotyledonous flowering plants, generally called as grasses. Piper betle L., also called as betel vine, is a member of the genus piper of the family piperaceae. Phenomenal health benefits of Triticum aestivum and piper betel for its antioxidant and anti-anemic activity have been systematically reported by Johri et al., presenting that the plants’ extract aids to assist its antioxidant properties. The numerous bio active compounds found in betel leaf and fruit indicate their ability to be utilized in a variety of medicinal processes [12]. To maintain good health, wheatgrass is ingested in India as a pill or juice. Wheat grass juice is well known for having therapeutic capabilities that can treat a variety of degenerative disorders. It is also highly helpful in treating thalassemia, ulcerative colitis, anemia, and other conditions that benefit various body regions. In this study, the molecular docking technique, which is quickly emerging as a key tool in pharmaceutical research, was used to predict the binding affinities of several synthetic and herbal components for active regions of hepcidin and transferrin [13-15]. Molecular docking is gaining importance in structure-based molecular genetics and computer aided drug development. By leveraging molecular docking technology to demonstrate how a little ligand and a protein interact at the nano scale, microscopic particles may be used to describe how significant biochemical processes take place and behave at the protein binding site. By applying various computational techniques and in-silico analyses, this work can be analyzed. Understanding how metabolites enter a receptor's binding site and figuring out the compound's binding affinity are both necessary for docking and identifying the druggable target. Genome annotation, proteomics experiment design, and the identification of new targets for vaccines, drugs, and diagnostics are also essential the entire proteome's Protein-Protein Interactions (PPI) or identifying the specific host-pathogen connection between a collection of proteins can be helpful in developing prospective therapeutic targets [16].
Data tracking
3 dimensional structural composition of potential protein targets both hepcidin, transferrin from the protein data bank were downloaded with their respective PDB ID’s in a folder. A total of two proteins were retrieved. CASTp11 was used to anticipate the proteins' active sites. Computed atlas of surface topography of proteins. Recent theoretical and algorithmic developments in computational geometry are the foundation of CASTp. It offers a lot of benefits:
• Analytical identification of pockets and cavities.
• Accurate definition of the border between the bulk solvent
and the pocket.
• Rotational invariance of all derived parameters, absence of
discretization, dot surface, or grid points.
The 2 dimensional structure of catechin, chlorogenic, 3, 4-dihydroxybenzaldehyde, folic acid, dasatinib, Fe(3+)-ascorbate, gluconate were retrieved from PubChem with their respective PubChem CID in a separate ligand folder.
Molecular docking studies
For understanding the proteins and ligands interactions, molecular docking analysis was practiced using the software’s PyRx-python prescription 0.8 in windows 10 home single language. Preparation of the protein-ligand interaction was made using auto dock tools-1.5.7 software. In the simulated space, the generation of the proteins hepcidin (1m4f) and transferrin (3qyt) includes adding polar hydrogens with unified atom kollman charges. Ligands (catechin, chlorogenic acid, 3, 4-dihydroxybenzaldehyde, folic acid, dasatinib, Fe(3+)-ascorbate, ferric gluconate) were prepared by adding polar hydrogens, along a gasteiger charge, with the detection of flexible torsions, and a setting for the number of torsions were added to prepare them. Based on the dimensions of each protein's active site, gridbox were described. Are used as the exhaustiveness factor to provide more consistent docking results. The optimum position with low affinity was chosen after manual interaction analysis and the maintenance of many conformations. Protein-ligand docking studies were done using the crystal structures of approved protein therapeutic targets.
The bio active compounds and synthetic drugs utilizing the Swiss-ADME software were simulated screened. To evaluate the molecules using the Lipinski rule and criteria for drug-likeness, such as pharmacokinetic parameters clearly shown in the Table 1 along with Table 2.
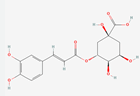
| Physicochemical properties | Catechin | Chlorogenic acid | 3,4-Dihydroxybenzaldehyde |
|---|---|---|---|
| Chemical structure | 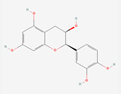 |
 |
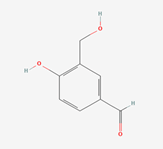 |
| Formula | C15H14O6 | C16H18O9 | C7H5NO5 |
| Molecular weight | 290.27 g/mol | 354.31 g/mol | 183.12 g/mol |
| Num heavy atoms | 21 | 25 | 13 |
| Num arom heavy atoms | 12 | 6 | 6 |
| Fraction Csp3 | 0.2 | 0.38 | 0 |
| Num. rotatable bonds | 1 | 5 | 2 |
| Num. H-bond acceptors | 6 | 9 | 5 |
| Num. H-bond donors | 5 | 6 | 2 |
| Molar refractivity | 74.33 | 83.5 | 44.7 |
| Topological polar surface area | 110.38 Ų | 164.75 Ų | 103.35 Ų |
| GI absorption | High | Low | Low |
| BBB permeant | No | No | No |
| P-gp substrate | Yes | No | No |
| CYP1A2 inhibitor | No | No | No |
| CYP2C19 inhibitor | No | No | No |
| CYP2C9 inhibitor | No | No | No |
| CYP2D6 inhibitor | No | No | No |
| CYP3A4 inhibitor | No | No | No |
| Log Kp (skin permeation) | -7.82 cm/s | -8.76 cm/s | -8.76 cm/s |
Table 1: Predicted physicochemical properties of natural ligands.
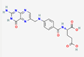
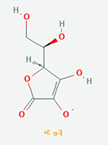
| Physico chemical properties | Dasatinib | Folic acid | Ferric-ascorbate | Ferric gluconate |
|---|---|---|---|---|
| Chemical structure | 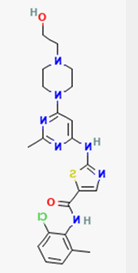 |
 |
 |
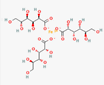 |
| Formula | C22H26ClN7O2S | C19H19N7O6 | C6H7FeO6++ | C18H33FeO21 |
| Molecular weight | 488.01 g/mol | 441.40 g/mol | 230.96 g/mol | 641.29 g/mol |
| Num. heavy atoms | 33 | 32 | 13 | 40 |
| Num. arom. heavy atoms | 17 | 16 | 0 | 0 |
| Fraction Csp3 | 0.36 | 0.21 | 0.5 | 0.83 |
| Num. rotatable bonds | 8 | 10 | 2 | 15 |
| Num. H-bond acceptors | 6 | 9 | 6 | 21 |
| Num. H-bond donors | 3 | 6 | 3 | 15 |
| Molar refractivity | 138.63 | 111.92 | 33.18 | 109.78 |
| Topological polar surface area | 134.75 Ų | 213.28 Ų | 110.05 Ų | 423.84 Ų |
| GI absorption | High | Low | High | Low |
| BBB permeant | No | No | No | No |
| P-gp substrate | No | No | Yes | Yes |
| CYP1A2 inhibitor | No | No | No | No |
| CYP2C19 inhibitor | Yes | No | No | No |
| CYP2C9 inhibitor | Yes | No | No | No |
| CYP2D6 inhibitor | Yes | No | No | No |
| CYP3A4 inhibitor | Yes | No | No | No |
| Log Kp (skin permeation) | -6.73 cm/s | -9.76 cm/s | -8.87 cm/s | -17.38 cm/s |
Table 2: Predicted physicochemical properties of synthetic ligands.
The investigated compounds are then subjected to molecular docking using auto dock tools (version 1.5.7) and PyRx-Python prescription 0.8 in a Windows 10 home single language workstation to properly evaluate their ability to bind to the iron-binding receptor, transferrin (3qyt), and iron ion transmembrane transporter inhibitor; signaling receptor, hepcidin (1m4f).
Data collection
In this paper, 2D structures of (catechin, chlorogenic acid, 3,4-dihydroxybenzaldehyde, folic acid, dasatinib, ferric gluconate, and 3+-ascorbate] were obtained from PubChem and drug bank sites. Two proteins in total-hepcidin (1m4f) and transferrin (3qyt) were downloaded from the protein data bank website.
Molecular docking
The binding energy of the compounds (catechin, chlorogenic acid, 3,4-dihydroxybenzaldehyde, folic acid, dasatinib, Fe(3+)-ascorbate, ferric gluconate) to the active site of target proteins was used to determine their affinity displayed in Table 3. So, using PyRx and AutoDockTools, seven ligands were docked to two proteins. The distribution of protein targets with high ligand binding affinity. The top seven ligands with high affinity. Further study was carried out on proteins with binding energies between 11 kcal/mol and 7 kcal/mol, and these proteins were considered as potential targets for protein-ligand interaction analyses.
| S. no. | Ligands | Binding affinity (kcal/mol) | RMSD/ub | RMSD/lb |
|---|---|---|---|---|
| 1. | Transferrin and dasatinib | -9 | 8.733 | 4.224 |
| 2. | Transferrin and follic acid | -8.1 | 2.213 | 1.691 |
| 3. | Transferrin and catechin | -7.2 | 3.003 | 1.818 |
| 4. | Transferrin and cholorogenic acid | -7 | 19.654 | 17.838 |
| 5. | Hepcidin and folic acid | -6.6 | 5.963 | 3.33 |
| 6. | Hepcidin and dasatinib | -5.8 | 20.904 | 20.418 |
| 7. | Transferrin and ferric ascorbate | -5.6 | 2.62 | 1.746 |
| 8. | Transferrin and benzaldehyde bihydroxy | -5.5 | 16.233 | 15.288 |
| 9. | Transferrin and ferric gluconate | -5.4 | 15.644 | 14.477 |
| 10. | Hepcidin and catechin | -4.9 | 7.085 | 2.973 |
| 11. | Hepcidin and cholorogenic acid | -4.8 | 6.59 | 1.786 |
| 12. | Hepcidin and ferric ascorbate | -4.4 | 2.802 | 2.428 |
| 13. | Hepcidin and ferric gluconate | -3.9 | 2.742 | 1.709 |
| 14. | Hepcidin and benzaldehyde bihydroxy | -3.5 | 3.336 | 1.741 |
Table 3: List of highly interacting ligands and their binding energies against hepcidin and transferrin where RMSD stands for Root-Mean-Square Deviation to calculate the rank as only moveable heavy atoms are used to compute RMSD values, which are generated in relation to the best mode (the first model) (i.e. only ligand atoms, not hydrogen). Each atom in one conformation is matched with itself in the opposite conformation by RMSD upper limit, which excludes any symmetry. Each atom in one conformation is paired with the nearest atom of the same element type in the opposite conformation using the RMSD lower bound.
Receptors-ligands interaction study
The protein data bank accession numbers 1m4f and 3qyt, transferrin, an iron receptor, and hepcidin, an iron ion transmembrane transporter inhibitor; signal receptor.
Following compound identification, only 7 compounds were chosen for molecular docking based on the virtual screening and their pharmacokinetic and pharmacodynamic characteristics. The targets were determined to be transferrin and hepcidin. When compared to the control medication, the two compounds with the highest binding affinities are catechin and chlorogenic acid with transferrin. Catechin showed serine and histidine with two different hydrogen bonds interacting with transferrin protein (Table 4). Chlorogenic acid demonstrated hydrogen bonds with two different amino acids: Two with arginine alone, one with glutamine, and two with methionine. In contrast, the control drug dasatinib demonstrated four hydrogen bonds with various amino acids: Tyrosine, lysine, glutamine, and methionine of the transferrin receptor displayed in Figures 1-13.
| Proteins | Functions |
|---|---|
| SLC40A1 | Solute carrier family 40 member 1; may be involved in iron export from duodenal epithelial cell and also in transfer of iron between maternal and fetal circulation. Mediates iron efflux in the presence of a ferroxidase (hephaestin and/or ceruloplasmin); belongs to the Ferro Portin (FP) (TC 2.A.100) family. |
| A2M | Alpha-2-macroglobulin; Is able to inhibit all four classes of proteinases by a unique 'trapping' mechanism. This protein has a peptide stretch, called the 'bait region' which contains specific cleavage sites for different proteinases. |
| HFE2 | Hemojuvelin; acts as a Bone Morphogenetic Protein (BMP) coreceptor. Through enhancement of BMP signaling regulates hepcidin (HAMP) expression and regulates iron homeostasis; belongs to the Repulsive Guidance Molecule (RGM) family. |
| HFE | Hereditary hemochromatosis protein; binds to Transferrin Receptor (TFR) and reduces its affinity for iron loaded transferrin; belongs to the MHC class I family. |
| TFR2 | Transferrin receptor protein 2; mediates cellular uptake of transferrin bound iron in a non-iron dependent manner. May be involved in iron metabolism, hepatocyte function and erythrocyte differentiation; belongs to the peptidase M28 family. |
| FAM132B | Erythroferrone; iron-regulatory hormone that acts as an erythroid regulator after hemorrhage: Produced by erythroblasts following blood loss and mediates suppression of hepcidin (HAMP) expression in the liver, thereby promoting increased iron absorption and mobilization from stores. Promotes lipid uptake into adipocytes and hepatocytes via transcriptional up-regulation of genes involved in fatty acid uptake. |
| SLC11A2 | Natural resistance associated macrophage protein 2; important in metal transport, in particular iron. Can also transport manganese, cobalt, cadmium, nickel, vanadium and lead. Involved in apical iron uptake into duodenal enterocytes. Involved in iron transport from acidified endosomes into the cytoplasm of erythroid precursor cells. May play an important role in hepatic iron accumulation and tissue iron distribution. May serve to import iron into the mitochondria. |
| TMPRSS6 | Transmembrane protease serine 6; serine protease which hydrolyzes a range of proteins including type I collagen, fibronectin and fibrinogen. Can also activate urokinase-type plasminogen activator with low efficiency. May play a specialized role in matrix remodeling processes in liver. Through the cleavage of HFE2, a regulator of the expression of the iron absorption regulating hormone hepicidin/HAMP, plays a role in iron homeostasis. |
| TFRC | Transferrin receptor protein 1; cellular uptake of iron occurs via receptor mediated endocytosis of ligand-occupied transferrin receptor into specialized endosomes. Endosomal acidification leads to iron release. The apotransferrin-receptor complex is then recycled to the cell surface with a return to neutral pH and the concomitant loss of affinity of apotransferrin for its receptor. Transferrin receptor is necessary for development of erythrocytes and the nervous system (by similarity). |
Table 4: Predicted functional partners of hepcidin.
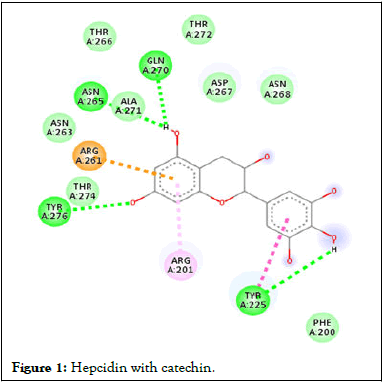
Figure 1: Hepcidin with catechin.
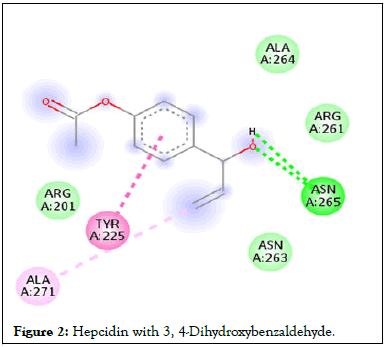
Figure 2: Hepcidin with 3, 4-Dihydroxybenzaldehyde.
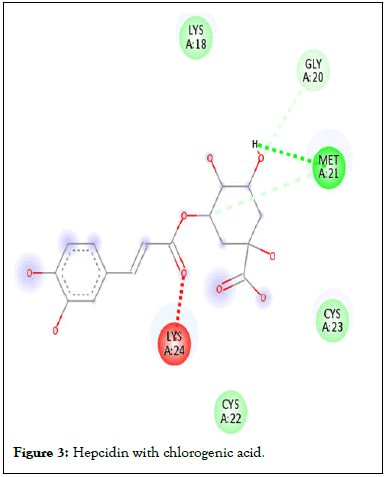
Figure 3: Hepcidin with chlorogenic acid.
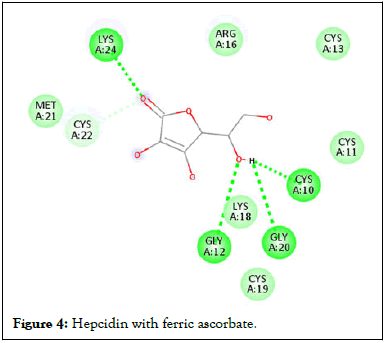
Figure 4: Hepcidin with ferric ascorbate.
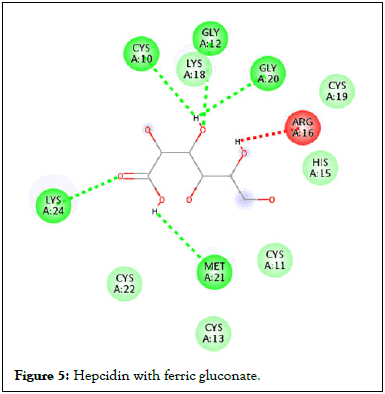
Figure 5: Hepcidin with ferric gluconate.
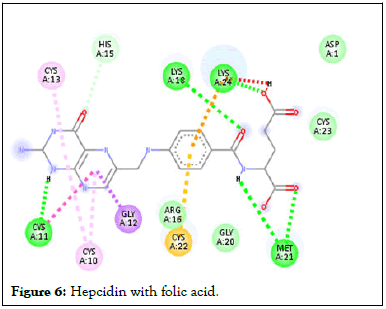
Figure 6: Hepcidin with folic acid.
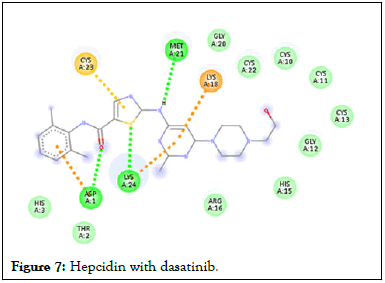
Figure 7: Hepcidin with dasatinib.
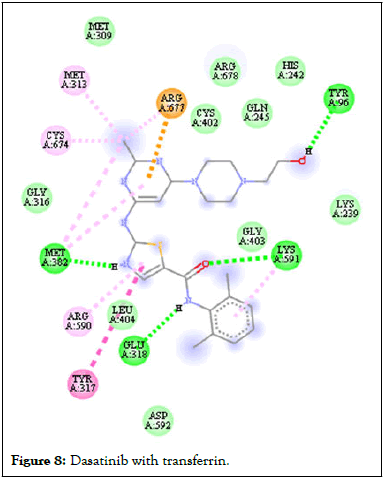
Figure 8: Dasatinib with transferrin.
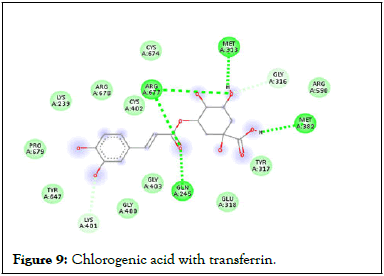
Figure 9: Chlorogenic acid with transferrin.
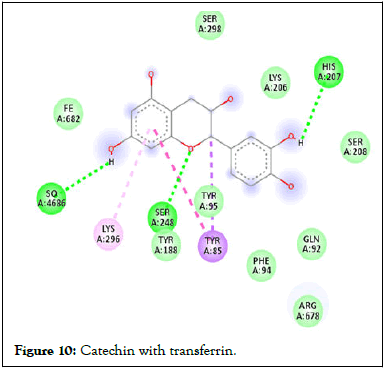
Figure 10: Catechin with transferrin.
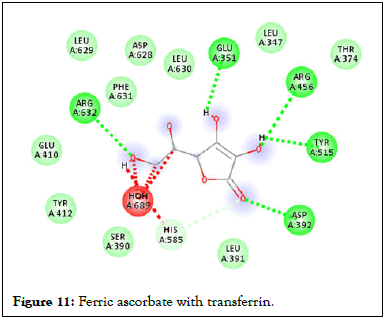
Figure 11: Ferric ascorbate with transferrin.
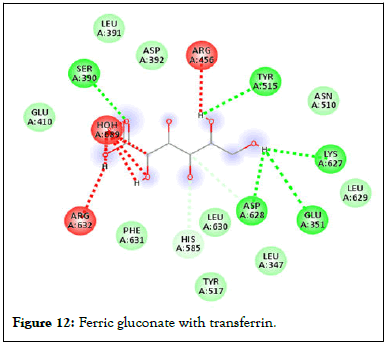
Figure 12: Ferric gluconate with transferrin.
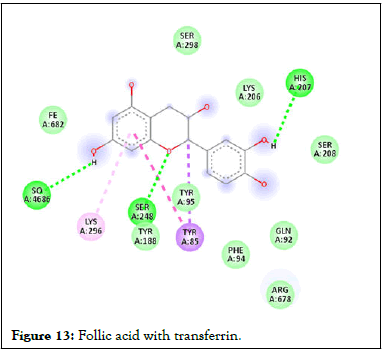
Figure 13: Follic acid with transferrin.
The physiological functions of the human body, including the delivery of oxygen to tissues and the storage and use of energy, depend heavily on iron. The proteins are divided into three categories based on their ligand affinities. The value of binding affinity, which ranges from 12 kcal/mol to 8 kcal/mol a as high affinity, defined. Low affinity is defined as binding affinity values less than 5 kcal/mol and moderate affinity as binding affinity values between 7.9 kcal/mol and 5 kcal/mol. New drug candidates are typically found using the Lipinski criteria, which include molecular weight, the number of H-bond donors present, the number of H-bond acceptors present, and the octanol-water partition coefficient (LogP). A molecular weight of less than 500 Da is advised. Smaller molecules require less energy to link together and form more durable bonds. A drug's capacity to pass a cell membrane is assessed using an H-bond donor and acceptor. The cell's permeability diminishes as the number of H-bonds rises. A compound's ability to dissolve in fat is determined by its logP or lipophilicity. Lower toxicity results from a compound's increased hydrophilicity when its lipophilicity declines [17]. Catechin and chlorogenic acid have demonstrated good binding affinity with the transferrin receptor in the current investigation, indicating a beneficial effect on the improvement of iron absorption. These bioactive agents can be found in the wheat grass and betel plant. It has been used to treat hyperglycemia, acute microbiological infection, a skin condition, inflammation, anemia, and local anesthetics (Tables 5-7) [18,19].
| Diseases | Matching proteins in the network of Hepcidin |
|---|---|
| Hemochromatosis | SLC40A1, HFE2, TFRC, TMPRSS6, SLC11A2, HFE, TFR2, HAMP |
| Hemochromatosis type 4 | SLC40A1, HFE2, HFE, TFR2, HAMP |
| Iron deficiency anemia | HFE2, TFRC, TMPRSS6, SLC11A2, HAMP |
| Acquired metabolic disease | A2M, HFE2, TFRC, TMPRSS6, SLC11A2, HFE, TFR2, HAMP |
| Hemochromatosis type 2 | HFE2, HFE,TFR2, HAMP |
| Disease of metabolism | SLC40A1, A2M, HFE2, TFRC, TMPRSS6, SLC11A2, HFE, TFR2, HAMP |
| Hemochromatosis type 1 | HFE2, HFE, TFR2 |
| Hemochromatosis type 3 | HFE2, HFE, TFR2 |
| Microcytic anemia | TMPRSS6, SLC11A2, HAMP |
| Anemia | EPO, TMPRSS6, SLC11A2, HFE, HAMP |
| Iron metabolism disease | HFE, TFR2, HAMP |
| Hypochromic microcytic anemia | TMPRSS6, HAMP |
| Atransferrinemia | HFE2, TFR2 |
| African iron overload | SLC40A1, HFE |
| Siderosis | HFE, HAMP |
| Hemosiderosis | HFE, HAMP |
Table 5: Disease-gene associations of Hepcidin.
| Proteins | Functions |
|---|---|
| TFRC | Transferrin receptor protein 1; cellular uptake of iron occurs via receptor mediated endocytosis of ligand occupied transferrin receptor into specialized endosomes. Endosomal acidification leads to iron release. The apotransferrin-receptor complex is then recycled to the cell surface with a return to neutral pH and the concomitant loss of affinity of apotransferrin for its receptor. Transferrin receptor is necessary for development of erythrocytes and the nervous system (by similarity). |
| APOA1 | Apolipoprotein A-I; participates in the reverse transport of cholesterol from tissues to the liver for excretion by promoting cholesterol efflux from tissues and by acting as a cofactor for the Lecithin Cholesterol Acyltransferase (LCAT). |
| SCG3 | Secretogranin iii; member of the granin protein family that regulates the biogenesis of secretory granules. Acts as a sorting receptor for intragranular proteins including chromogranin A/CHGA (By similarity). May also play a role in angiogenesis. |
| SYNJ1 | Synaptojanin-1; phosphatase that acts on various phosphoinositides, including phosphatidylinositol 4-phosphate, phosphatidylinositol (4,5)-bisphosphate and phosphatidylinositol (3,4,5)-risphosphate. |
| TFR2 | Transferrin receptor protein 2; mediates cellular uptake of transferrin bound iron in a non-iron dependent manner. May be involved in iron metabolism, hepatocyte function and erythrocyte differentiation; belongs to the peptidase M28 family. M28B subfamily. |
| AHSG | Alpha-2-HS-glycoprotein; promotes endocytosis, possesses opsonic properties and influences the mineral phase of bone. |
| AMBP | Alpha-1-microglobulin/bikunin precursor; protein AMBP; Inter-alpha-trypsin inhibitor inhibits trypsin, plasmin, and lysosomal granulocytic elastase. Inhibits calcium oxalate crystallization; lipocalins. |
| ALB | Serum albumin; serum albumin, the main protein of plasma, has a good binding capacity for water, Ca(2+), Na(+), K(+), fatty acids, hormones, bilirubin and drugs. Its main function is the regulation of the colloidal osmotic pressure of blood. Major zinc transporter in plasma, typically binds about 80% of all plasma zinc; belongs to the ALB/AFP/VDB family. |
| APOA2 | Apolipoprotein A-II; may stabilize HDL (High Density Lipoprotein) structure by its association with lipids, and affect the HDL metabolism; apolipoproteins. |
| HP | Haptoglobin-related protein; haptoglobin; As a result of hemolysis, hemoglobin is found to accumulate in the kidney and is secreted in the urine. Haptoglobin captures, and combines with free plasma hemoglobin to allow hepatic recycling of heme iron and to prevent kidney damage. Haptoglobin also acts as an antimicrobial; antioxidant has antibacterial activity and plays a role in modulating many aspects of the acute phase response. |
Table 6: Predicted functional partners of transferrin.
| Diseases | Matching proteins in transferrin network |
|---|---|
| Disease of metabolism | APOC3, APOB, APOA1, LCAT, ALB, HP, TFRC, APOA2, ABCA1, AHSG, HFE, TFR2, B2M |
| Acquired metabolic disease | APOC3, APOA1, ALB, TFRC, APOA2, AHSG, HFE, TFR2, B2M |
| Familial visceral amyloidosis | APOC3, APOA1, ALB, APOA2, B2M |
| Hypolipoproteinemia | APOB, APOA1, LCAT, ABCA1 |
| Lipid metabolism disorder | APOC3, APOB, APOA1, LCAT, ABCA1 |
| Inherited metabolic disorder | APOC3, APOB, APOA1, LCAT, HP, TFRC, ABCA1, HFE, TFR2 |
| Tangier disease | APOA1, LCAT, ABCA1 |
| Artery disease | FCGRT, APOB, APOA1, ALB, ABCA1 |
| Hemochromatosis | TFRC, HFE, TFR2 |
| Atherosclerosis | APOB, APOA1, ABCA1 |
| Norum disease | APOA1, LCAT |
| Mineral metabolism disease | AHSG, HFE, TFR2 |
| Hemochromatosis type 1 | HFE, TFR2 |
| Hemochromatosis type 3 | HFE, TFR2 |
| Familial combined hyperlipidemia | APOB, APOA1 |
| Hemochromatosis type 4 | HFE, TFR2 |
| Viral hepatitis | ALB, AHSG |
| Hemochromatosis type 2 | HFE, TFR2 |
| Iron metabolism disease | HFE, TFR2 |
| Disease | FCGRT, APOC3, APOB, APOA1, LCAT, ALB, HP, TFRC, APOA2, ABCA1, AHSG, SYNJ1, HFE, TFR2, B2M |
| Proteinuria | ALB, HP |
Table 7: Disease-gene associations of transferrin.
These two plants, Piper betel Linn and Triticum aestivum Linn, were chosen because of their greater and more suggestive iron concentration. It has been noted that there are significantly more bio active compounds in the extracts of Piper betel and Triticum aestivum Linn. When compared to the control medication, two of the discovered compounds catechin and chlorogenic acid with transferrin showed a greater binding affinity with the iron-binding receptor.
The authors would like to thank Dr. Prashanth N Suravajhala, founder of Bioinformatics Cub for experimenting scientists (BIOCLUES) for guiding with the molecular docking analysis.
The author has no conflicts of interest.
Sonia Johri drafted the paper along with the interpretation of the data related to protein-protein interaction. Nabomita Paul wrote the manuscript and performed molecular docking along with protein-protein interaction network. Sonia Johri and Neha Khan compiled the review and preliminary studies. All authors approved the manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sonia J, Nabomita P, Neha K (2023) Pharmacokinetic Attributes for Natural and Chemical Anti-Anemic Agents with Promising Specific Proteins by Docking Analysis. J Theor Comput Sci. 9:198.
Received: 24-Apr-2023, Manuscript No. JTCO-23-23719; Editor assigned: 26-Apr-2023, Pre QC No. JTCO-23-23719 (PQ); Reviewed: 10-May-2023, QC No. JTCO-23-23719; Revised: 22-Jun-2023, Manuscript No. JTCO-23-23719 (R); Published: 28-Dec-2023 , DOI: 10.35248/2376-130X.23.9.206
Copyright: © 2023 Sonia J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.