Chemotherapy: Open Access
Open Access
ISSN: 2167-7700
ISSN: 2167-7700
Research Article - (2023)Volume 11, Issue 1
Background: Compared with conventional mitoxantrone, liposomal mitoxantrone hydrochloride (PLM60) has shown promising antineoplastic effect and better safety profiles in the previous studies, and worth a further evaluation on its pharmacokinetic profiles.
Methods: In this single-center, open-label, randomized, parallel-group study, patients with histologically/cytologically confirmed relapse/refractory non-Hodgkin lymphoma (n=18) and Hodgkin lymphoma (n=6) were randomized to receive PLM60 12 mg/m2, 16 mg/m2 and 20 mg/m2 on day 1 of each 28-day cycle until the completion of 4 cycles treatment, disease progression, intolerable toxicities, or patient/investigator decision to withdraw (whichever occurred first). Blood samples were collected at prespecified timepoint and the primary endpoint was the pharmacokinetic parameters of total mitoxantrone and free mitoxantrone, the second endpoint was the incidence of adverse event and severe adverse event during the treatment, as well as Overall Response Rate (ORR) and Progression Free Survival (PFS) after PLM60 treatment.
Results: Of 32 patients screened, 24 patients were enrolled between July 28, 2019 and June 22, 2020. Cmax, AUC0-t and AUC0→∞ of total mitoxantrone and free mitoxantrone increased with the increasing doses, and both showed liner pharmacokinetics profiles. The ratio of mean AUC0-t and AUC0-∞ of free mitoxantrone to total mitoxantrone was 0.94%, 1.23%, 0.98% and 0.98%, 1.24%, 0.99%, separately. All the patients completed at least 1 cycle of treatment except that one patient in 12 mg/m2 group discontinued treatment due to hypersensitivity of which, 15 patients completed 4 cycles of treatment. All 24 patients experienced treatment related adverse events (TRAE). The most common (≥ 5%) TRAE was leucopenia, neutropenia, thrombocytopenia, anemia and skin hyperpigmentation. Leucopenia and neutropenia were grade 3 or 4 TRAE occurring ≥ 5% patients. After a median 5.6 month of follow- up, ORR and disease control rate of 24 patients was 41.7% (10/24, 95% CI, 22.1%-63.4%) and 62.5% (15/24, 95% CI, 40.6%-81.2%) respectively, with 2 patients achieving complete remission. Median PFS was 7.6 month (95% CI 3.2-NA).
Conclusion: After administering 12 mg/m2-20 mg/m2, PLM60 had a favorable pharmacokinetic profile as a liposomal preparation and showed preliminary efficacy in patients with relapse/refractory lymphoma with manageable safety.
Liposomal mitoxantrone hydrochloride; Pharmacokinetic; Efficacy; Safety; Relapse/refractory advanced lymphoma
AITL: Angioimmunoblastic T-cell Lymphoma; BSA: Body Surface Area; CLL/SLL: Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma; DBCLC: Diffuse Large B-cell Lymphoma; ECOG: Eastern Cooperative Oncology Group; FL: Follicular Lymphoma; HL: Hodgkin Lymphoma; LVEF: Left Ventricular Ejection Fraction; MZL: Marginal Zone Lymphoma; NHL: Non-Hodgkin Lymphoma; SD: Standard Deviation.
Lymphoma is one of common malignancies in China, according to recent paper by Zhang et al., there are 90 000 new cases diagnosed in China, with an incidence rate of 6.52/10 000, and there are geographic different in the lymphoma incidence rate, rural-urban difference is observed as well [1]. Chemotherapy still plays an important role in the treatment of non-Hodgkin lymphoma and Hodgkin lymphoma [2]. For these patients with relapse/refractory lymphoma, 2021 CSCO guideline recommends to trying novel therapy in clinical trial (grade I or II) based on the tumor condition and patient performance status [3].
Mitoxantrone is an anthracycline antibiotic and a DNA topoisomerase II inhibitor, used for the treatment of lymphoma, acute leukemia and some kind of solid tumor [4]. However, its use had been limited due to some toxic effects, including bone marrow suppression and cardiotoxicity. Encapsulation the antitumor drug with liposome resulted in increased vesicle size of the drug, therefore passively targeted the tumor through enhanced permeability [5]. Some liposomal preparations of antitumor drugs such as liposomal doxorubicin and liposomal irinotecan have been introduced successfully into clinical practice, however, till now no liposomal preparations of mitoxantrone is available worldwide.
Many efforts had been made to develop liposomal preparation of mitoxantrone during the last decades [6-9], none of which got the chance to be tested in clinical trials except Liposome-Complexed Mitoxantrone (LCM). While Pestalozzi et al. [10] evaluated the efficacy and safety of LCM after treating advanced breast cancer patients with four cycles in a phase I/Ⅱ clinical study, they found that due to drug leakage from the liposomal bilayer and inadequate circulation time, LCM didn’t have advantages over conventional mitoxantrone on efficacy and safety results. Thus it’s essential for liposomal preparations to ensure the free drug released steadily in the target tissues.
Liposomal mitoxantrone hydrochloride (PLM60, CSPC Zhongqi Pharmaceutical Technology [Shijiazhuang] Co., Ltd.) in our study used PEG-modified liposome, with vesicle size of 60 nm, which could allow more mitoxantrone release after the accumulation into tumor zone [11], and exhibited improved efficacy profiles. Previous studies demonstrated that compared with conventional mitoxantrone, PLM60 had shown improved pharmacokinetics profiles and tissue distribution, characterized as the higher peak concentration and AUC in the tumor tissues, while lower peak concentration and AUC in the normal tissues (heart, kidney, lung, spleen and intestinal issue), thus increased the therapeutic effect and safety profiles [12,13].
Thus, to further explore the pharmacokinetic characteristics of PLM60 developed by CSPC, we conducted this study in patients with relapse/refractory non-Hodgkin lymphoma and Hodgkin lymphoma, especially analyzed the proportion of plasma concentration of free mitoxantrone to total mitoxantrone after PLM60 administration.
Study design and patients
This single-center, open-label, randomized, parallel-group study was aimed to assess the pharmacokinetics, safety and tolerability, preliminary anti-tumor activity of PLM60 in patients with relapse/ refractory lymphoma. Patients were screened within 28 days prior to treatment period and all the eligible patients were randomized patients were randomized (1:1:1) to receive intravenous infusion of 12 mg/m2, 16 mg/m2 or 20 mg/m2 of PLM60 (10 mg/10 ml, CSPC Zhongqi Pharmaceutical Technology [Shijiazhuang] Co., Ltd.) on day 1 of each 28-day cycle, until the completion of 4 cycles treatment, disease progression, intolerable toxicity, or patient/investigator decision to withdraw (whichever occurred first). The block randomization was used to assign the patients to each dosage group by random coding table generated from SAS version 9.4.
Patients were eligible if they 1) Had a histologically/cytologically confirmed relapse/refractory non-Hodgkin lymphoma and Hodgkin lymphoma, 2) Failed 1st line standard treatment or had recurred after 1st line standard treatment, 3) Advanced lymphoma without available treatment, 4) Aged between 18 and 70 years, 5) Had an Eastern Cooperative Oncology Group performance status of 0-2 with life expectancy of 3 months or more, 6) Had adequate organ function (White Blood Cell Count [WBC] ≥ 3.5 × 109/L, Absolute Neutrophil Count [ANC] ≥ 1.5 × 109/L, Platelet Cell Count [PLT] ≥ 75 × 109/L, hemoglobin concentration [Hb] ≥ 90 g/L, total bilirubin ≤ 1.5 times the Upper Limit Of Normal [ULN], alanine aminotransferase and aspartate aminotransferase ≤ 2.5 times ULN). Patients were excluded if they had received previous treatment of conventional mitoxantrone or liposomal mitoxantrone, or had received previous treatment of doxorubicin or other anthracycline medications with a cumulative dose of up to 360 mg/m2 (for doxorubicin, dose conversion coefficient: 1 of doxorubicin=2 of epirubin=2 of daunorubin=0.5 of Idarubin) [14]. Other exclusive criteria included: a history or concurrent other malignancy except for adequately treated basal cell carcinoma of the skin and cancer of the cervix in situ; intracranial lesions or history of brain metastases; clinically significant cardiac dysfunction; received anti-tumor treatment (including chemotherapy, radiotherapy, hormonal therapy, or herbal medicine with anti-tumor activity) within 4 weeks before the first dose.
The study protocol and informed consent were approved by the ethic committee of Affiliated Cancer Hospital of Guizhou Medical University; this study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. Written informed consents had been obtained from all the patients before the enrollment (NCT05173545)
Treatment
Before each treatment cycle, Body Surface Area (BSA) was estimated based on Stevenson’s formula: BSA(m2)=0.0061 × height(cm)+0.0128 × weight(kg)-0.1529, and therefore calculated or adjusted the PLM60 dose for individual patient [15]. PLM60 injection was added to 250 mL of 5% glucose and the solution should be given over 60 minutes under the supervision of the physician. Discontinuation of PLM60 and other related management was at the discretion of the physician if local infusion related reaction or other severe adverse effects occurred during the study. No dose escalation or reduction was allowed.
Patients could continue the treatment only when they met the following criteria: ANC ≥ 1.5 × 109/L, PLT ≥ 75 × 109/L, Hb ≥ 80 g/L (ANC could be extended to ≥ 1.0 × 109/L, PLT could be extended to ≥ 50 × 109/L, Hb could be extended to ≥ 75 g/L if there was bone marrow involvement); Once non-hematologic toxicities (excluding alopecia) occurred, the symptoms had to resolve to grade 1 or baseline level. After four weeks when the last patient has received the last dosing of PLM60, the study was formally closed.
Pharmacokinetic assessment
Blood samples were collected in K2EDTA anticoagulant tubes for the analysis of total mitoxantrone and free mitoxantrone at the following time point: 0.5 h before dosing and during the 60 minutes administration of the study drug, 0.5, 1, 2, 6, 12, 24, 48, 96, 144, 216, 288, and 360 hours post dose on day 1 of the first cycle, as well as 0.5 h before dosing of the second cycle. Plasma concentration of total mitoxantrone and free mitoxantrone were determined using High Performance Liquid Chromatography- Tandem Mass Spectrometric Detection (HPLC-MS/MS) method (lower limit of quantification of 50.0 ng/mL and 1.00 ng/mL, respectively).
Mean plasma concentration-time curve was generated based on the Pharmacokinetic Concentration Set (PKCS), who have received at least one dose of PLM60 and have at least one drug concentration result. The main pharmacokinetic parameter of total mitoxantrone and free mitoxantrone were estimated using noncompartmental model (Phoenix WinNonlin version 8.1) based on Pharmacokinetic Parameter Set (PKPS), who have received at least one dose of PLM60 and have at least one available pharmacokinetic parameter, including maximum concentration in plasma (Cmax), time to maximum concentration (Tmax), area under the plasma concentration-time curve from time zero to the last measurable concentration (AUC0-t), area under the plasma concentration-time curve from time zero to infinity (AUC0-∞), terminal elimination half-life (t1/2), apparent Volume Of Distribution (Vd), renal clearance (CL). The ratio of AUC0-t, AUC0-∞, and plasma concentration of free mitoxantrone to total mitoxantrone were calculated. Dose-proportional relationship between dose and AUC0-t, AUC0-∞, Cmax of total mitoxantrone and free mitoxantrone was analyzed by power function model described below: ln(Y)=α+β•ln(dose) and the linear pharmacokinetic profiles was indicated when the 90% confidence internal (CI) of the value β contain 1.00 [16].
Safety assessment
Safety was evaluated at baseline and day 1, day 7, day 16, day 21 and day 28 of each cycle, up to the end of the study. Safety assessment included laboratory tests, vital signs, physical examination, 12-led ECG, echocardiogram, and adverse events recording.
Safety was analyzed based on Safety Analysis Set (SS), who have received at least one dose of PLM60 and have at least one safety recording after administration. All Adverse Events (AEs) were recorded and coded by MedDRA (23.1 version). The incidence, severity of Adverse Events (AEs) and Treatment Related Adverse Events (TRAE) were assessed according to CTCAE version 4.03.
Efficacy assessment
Tumor assessment was carried out using enhanced CT scan (covering neck, thorax, abdomen, and pelvis), bone marrow aspiration and/or biopsy during screening (within 28 days before randomization) and day 28 of cycle 2 and cycle 4, and every 8 weeks after completion of the treatment. All the imaging was evaluated by the same experienced radiologist, and reviewed by independent radiologic committee. Response was assessed according to Cheson 2007 criteria and the primary efficacy endpoint was Overall Response Rate (ORR), which was defined as the proportion of patients Achieving Complete Response (CR) and Partial Response (PR) throughout the study, the second efficacy endpoint was Progression-Free Survival (PFS), which was defined as the time from first dosing to Disease Progression (PD) or dearth from any cause [17].
Based on Full Analysis Set (FAS), who were randomized to treatment and have received at least one dose of PLM60, ORR was calculated with 95% CI using Clopper Pearson method. Kaplan- Meier method was used to estimate median PFS and 95% CI.
Patients
32 patients were screened between July 29 2019 and June 22 2020, of which 8 patients failed and finally 24 patients enrolled in this study. The main reason for screening failure was not met inclusion criteria (n=5), met exclusion criteria (n=2) and both (n=1) (Figure 1).
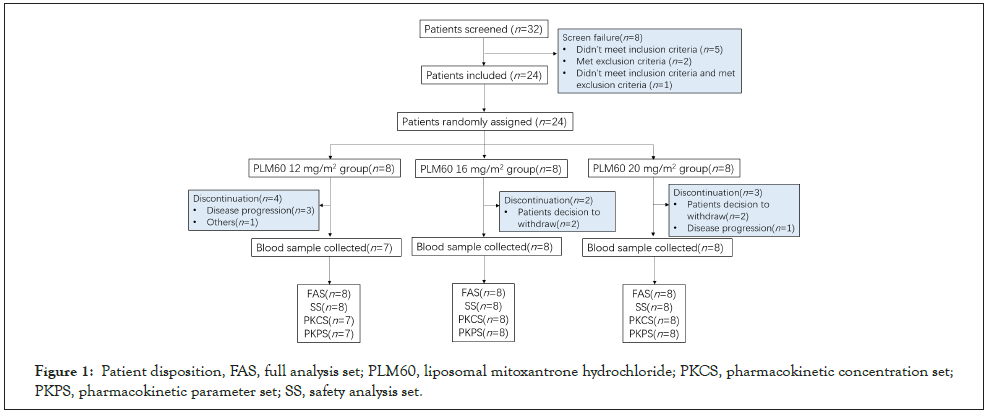
Figure 1: Patient disposition, FAS, full analysis set; PLM60, liposomal mitoxantrone hydrochloride; PKCS, pharmacokinetic concentration set; PKPS, pharmacokinetic parameter set; SS, safety analysis set.
Patient demographics and baseline disease characteristics are summarized in Table 1. Of 24 patients enrolled, 18(75.0%) were male, and the median age was 53.0 years (range: 18~70), median time from initial lymphoma diagnose was 18.20 months (range 8.0- 74.5). All 24 patients received previous treatment of anthracycline medications, and the majority (13/18, 72.2%) of patients with non- Hodgkin lymphoma had received rituximab-containing regimen (Table 1).
| PLM60 12 mg/m2 n=8 |
PLM60 16 mg/m2 n=8 |
PLM60 20 mg/m2 n=8 |
Total n=24 | |
|---|---|---|---|---|
| Age(y), Median (range) | 52.5 (33~70) | 48.5 (18~67) | 56.5 (51~70) | 53.0 (18~70) |
| Male, n (%) | 6 (75.0) | 6 (75.0) | 6 (75.0) | 18 (75.0) |
| Race, n (%) | ||||
| Han | 5 (62.5) | 4 (50.0) | 8 (100.0) | 17 (70.8) |
| Others | 3 (37.5) | 4 (50.0) | 0 (0.0) | 7 (29.2) |
| BSA(m2), Mean ± SD | 1.54 ± 0.15 | 1.58 ± 0.14 | 1.55 ± 0.13 | 1.56 ± 0.14 |
| ECOG score, 0/1/2, n | 01-07-2000 | 0/8/0 | 0/7/1 | 1/22/1 |
| Median time from initial diagnosis (m) (range) Baseline disease |
24.45 (9.3~74.5) | 20.45 (8.3~73.6) | 15.15 (8.0~34.0) | 18.20 (8.0~74.5) |
| HL | 3 (37.5) | 2 (25.0) | 1 (12.5) | 6 (25.0) |
| NHL | 5 (62.5) | 6 (75.0) | 7 (87.5) | 18 (75.0) |
| DLBCL | 4 | 4 | 4 | 12 |
| CLL/SLL | 1 | 0 | 1 | 2 |
| FL | 0 | 1 | 0 | 1 |
| AITL | 0 | 1 | 0 | 1 |
| MZL | 0 | 0 | 1 | 1 |
| Others | 0 | 0 | 1 | 1 |
| Disease stage at diagnosis | ||||
| I | 1 (12.5) | 1 (12.5) | 2 (25.0) | 4 (16.7) |
| II | 2 (25.0) | 0 (0.0) | 1 (12.5) | 3 (12.5) |
| III | 3 (37.5) | 5 (62.5) | 2 (25.0) | 10 (41.7) |
| IV | 2 (25.0) | 2 (25.0) | 2 (25.0) | 6 (25.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (4.1) |
| Prior therapy, n (%) | ||||
| Radiotherapy | 2 (25.0) | 5 (62.5) | 1 (12.5) | 8 (33.3) |
| Systemic therapy | 8 (100.0) | 8 (100.0) | 8 (100.0) | 24 (100.0) |
| Disease status relative to previous therapy, n (%) | ||||
| Relapse | 7 (87.5) | 8 (100.0) | 6 (75.0) | 21 (87.5) |
| Refractory | 1 (12.5) | 0 (0.0) | 2 (25.0) | 3 (12.5) |
| LVEF at baseline (%), Mean ± SD | 62.8 ± 3.15 | 63.8 ± 4.06 | 58.4 ± 3.70 | 61.6 ± 4.23 |
Table 1: Demographics and baseline disease characteristics.
Pharmacokinetics
As shown in Figure 2a, and Figure 2b, that plasma concentration of total mitoxantrone and free mitoxantrone increased with increasing PLM60 doses from 12 to 20 mg/m2, the curve increased rapidly at the absorption period and then declined gradually to a minimal concentration before the administration of the second dosing (~672 h after infusion) (Figure 2).
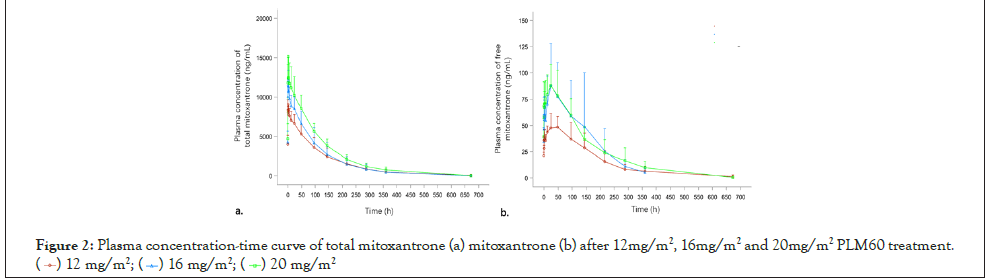
Figure 2:Plasma concentration-time curve of total mitoxantrone (a) mitoxantrone (b) after 12mg/m2, 16mg/m2 and 20mg/m2 PLM60 treatment.
The ratio of plasma concentration of free mitoxantrone to total mitoxantrone were shown in Figure 3,while the ratios of AUC0-t and AUC0-∞ of free mitoxantrone to total mitoxantrone were calculated as 0.94%, 1.23%, 0.98% and 0.98%, 1.24%, 0.99% for 12 mg/m2, 16 mg/m2 and 20 mg/m2 group, respectively, indicated that there was few free mitoxantrone released in the circulation after PLM60 infusion, and the ratio was fixed with ~1% (Figure 3).
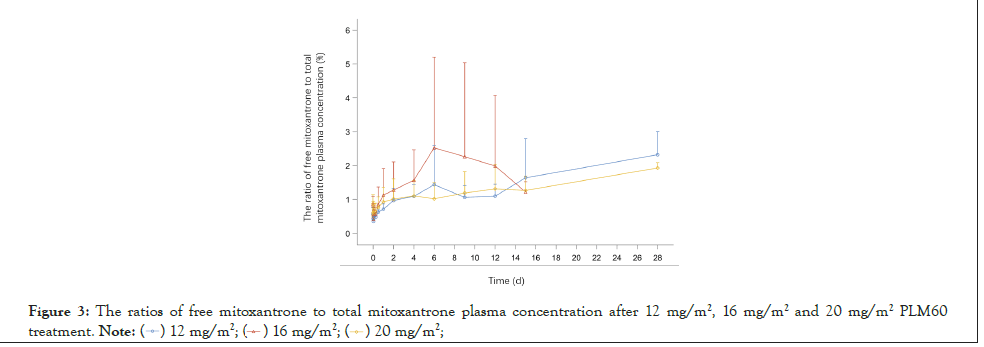
Figure 3:The ratios of free mitoxantrone to total mitoxantrone plasma concentration after 12 mg/m2, 16 mg/m2 and 20 mg/m2 PLM60 treatment.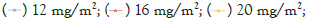
Following 60-minte infusion, Cmax of free mitoxantrone was reached with a mean time of 21.64-28.85 hours, in contrast with 1.92-2.02 hours that Cmax of total mitoxantrone reached, which suggested that free mitoxantrone was released slowly from PLM60 into the circulation. The mean Vd of total mitoxantrone was 1287.10~1569.73 mL/m2, and since the average Chinese adult BSA is 1.6 m2, indicated that PLM60 existed mainly as liposome encapsulated mitoxantrone in the circulation. The detailed pharmacokinetic parameter of total mitoxantrone and free mitoxantrone were shown in Table 2.
| Dose | Parameter | Total mitoxantronea | Free mitoxantroneb | ||
|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | ||
| 12 mg/m2 n=7 |
Cmax(ng/mL) | 8707.14 | 13.4 | 51.76 | 22.3 |
| Tmax(h) | 1.92 | 1.00~3.00 | 28.85 | 2.00~49.03 | |
| AUC0-t (h*μg/mL) | 1024.79 | 38.1 | 9.64 | 38.6 | |
| AUC0-ꝏ (h*μg/mL) | 1045.9 | 37.7 | 10.27 | 38.7 | |
| t1/2(h) | 81.95 | 46.63~119.32 | 122.64 | 31.28~203.26 | |
| Vd (mL/m2 or L/m2) | 1392.22 | 13.1 | 207.86 | 36.1 | |
| CL (mL/h/m2 or L/h/m2) | 13.13 | 41 | 1.3 | 30.7 | |
| 16 mg/m2 n=8 |
Cmax(ng/mL) | 11615 | 16 | 93.04 | 39 |
| Tmax(h) | 2.02 | 1.50~3.00 | 28.26 | 1.07~97.13 | |
| AUC0-t (h*μg/mL) | 1140.67 | 38.1 | 14.05 | 59.1 | |
| AUC0-ꝏ (h*μg/mL) | 1185 | 38.9 | 14.7 | 56.8 | |
| t1/2 (h) | 67.69 | 16.01~102.70 | 70.74 | 24.84~103.77 | |
| Vd (mL/m2 or L/m2) | 1287.1 | 18.7 | 139.16 | 44.4 | |
| CL (mL/h/m2 or L/h/m2) | 16.14 | 51.7 | 1.41 | 54.8 | |
| 20 mg/m2 n=8 |
Cmax(ng/mL) | 12848.75 | 21.4 | 96.1 | 22.6 |
| Tmax(h) | 1.96 | 1.05~3.00 | 21.64 | 2.00~49.08 | |
| AUC0-t (h*μg/mL) | 1507.28 | 22.1 | 14.71 | 38.7 | |
| AUC0-ꝏ (h*μg/mL) | 1573.26 | 21.3 | 15.59 | 34.7 | |
| t1/2 (h) | 84 | 56.51~104.22 | 90.84 | 53.74~130.54 | |
| Vd (mL/m2 or L/m2) | 1569.73 | 19.6 | 178.97 | 37 | |
| CL (mL/h/m2 or L/h/m2) | 13.22 | 21.9 | 1.38 | 25.5 | |
Table 2: Pharmacokinetic parameters of total mitoxantrone and free mitoxantrone in patients receiving 12-20 mg/m2 PLM60 treatment. Note: aThe units for total mitoxantrone Vd and CL after plm60-s therapy were mL/m2 and mL/h/m2, respectively. bThe units for free mitoxantrone Vd and CL after plm60-s therapy were L/m2 and L/h/m2, respectively.
Power function model analysis showed that, 90% CI for β value of Cmax, AUC0-t and AUC0-∞ of free mitoxantrone and total mitoxantrone were 0.746-1.701, 0.117-1.557, 0.132-1.557 and 0.429-1.051, 0.198-1.466, 0.230-1.520, all containing value 1, which indicated that free mitoxantrone and total mitoxantrone showed linear pharmacokinetics profiles over dose range 12 mg/m2 to 20 mg/m2 (Figures 4) (Table 3).
Figure 4:Relationship between the extent of exposure and dose of PLM60. (a)Cmax, (b)AUC0-t, and (c)AUC0-∞ of total mitoxantrone versus dose after 12 mg/m2, 16 mg/m2 and 20 mg/m2 PLM60 treatment; (d) Cmax, (e) AUC0-t, and (f) AUC0-∞ of free mitoxantrone versus dose after 12 mg/m2, 16 mg/m2 and 20 mg/m2 PLM60 treatment.
| Parameter | Estimated values | 90% confidence interval |
|---|---|---|
| Parameter of total mitoxantrone | ||
| Cmax | ||
| α | 7.249 | 6.387~8.111 |
| β | 0.74 | 0.429~1.051 |
| AUC0-t | ||
| α | 4.751 | 2.994~6.508 |
| β | 0.832 | 0.198~1.466 |
| AUC0-ꝏ | ||
| α | 4.666 | 2.879~6.452 |
| β | 0.875 | 0.230~1.520 |
| Parameter of free mitoxantrone | ||
| Cmax | ||
| α | 0.954 | -0.368~2.276 |
| β | 1.224 | 0.746~1.701 |
| AUC0-t | ||
| α | 0.149 | -1.845~2.143 |
| β | 0.837 | 0.117~1.557 |
| AUC0-ꝏ | ||
| α | 0.187 | -1.786~2.160 |
| β | 0.845 | 0.132~1.557 |
Table 3: Dose proportionality assessment of total mitoxantrone and free mitoxantrone in patients receiving 12-20 mg/m2 PLM60 treatment.
Safety
24 patients were included in SS for safety analysis, all the patients completed at least 1 cycle of treatment except that one patient in 12 mg/m2 group discontinued treatment due to hypersensitivities after administration of the first dose. Of which, 15 (62.5%) patients completed 4 cycles of treatment (n=4 for 12 mg/m2 group, n=6 for 16 mg/m2 group, n=5 for 20 mg/m2 group). Mean exposure dose was 50.65 ± 23.30 mg/m2, and mean exposure duration was 63.90 ± 36.75 days.
All 24 patients experienced treatment related adverse events (TRAE). The most common TRAE was leucopenia, neutropenia, anemia, thrombocytopenia and skin hyperpigmentation (Table 4).
| TRAE | PLM60 12 mg/m2 n=8 |
PLM60 16 mg/m2 n=8 |
PLM60 20 mg/m2 n=8 |
Total n=24 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade n(%) | ≥ Grade 3 n(%) |
Any grade n(%) |
≥ Grade 3 n(%) |
Any grade n(%) |
≥ Grade 3 n(%) |
Any grade n(%) |
≥ Grade 3 n(%) |
|||||
| Hematologic | ||||||||||||
| Leucopenia | 6 (75.0%) | 1 (12.5%) | 7 (87.5%) | 6 (75.0%) | 6 (75.0%) | 3 (37.5%) | 19 (79.2%) | 10 (41.7%) | ||||
| Neutropenia | 4 (50.0%) | 2 (25.0%) | 6 (75.0%) | 6 (75.0%) | 5 (62.5%) | 4 (50.0%) | 15 (62.5%) | 12 (50.0%) | ||||
| Anemia | 3 (37.5%) | 0 | 3 (37.5%) | 1 (12.5%) | 5 (62.5%) | 0 | 11 (45.8%) | 1 (4.2%) | ||||
| Thrombocytopenia | 4 (50.0%) | 0 | 3 (37.5%) | 0 | 4 (50.0%) | 0 | 11 (45.8%) | 0 | ||||
| Non-Hematologic | ||||||||||||
| Skin hyperpigmentation | 1 (12.5%) | 0 | 5 (62.5%) | 0 | 5 (62.5%) | 0 | 11 (45.8%) | 0 | ||||
| Urine occult blood test positive | 1 (12.5%) | 0 | 0 (0.0%) | 0 | 1 (12.5%) | 0 | 2 (8.3%) | 0 | ||||
| Abnormal ECG | 1 (12.5%) | 0 | 0 (0.0%) | 0 | 1 (12.5%) | 0 | 2 (8.3%) | 0 | ||||
| Palpitation | 1(12.5%) | 0 | 0 (0.0%) | 0 | 1(12.5%) | 0 | 2 (8.3%) | 0 | ||||
| Hypoproteinemia | 1 (12.5%) | 0 | 1 (12.5%) | 0 | 0 (0.0%) | 0 | 2 (8.3%) | 0 | ||||
| Iron deficiency | 0 (0.0%) | 0 | 1 (12.5%) | 0 | 1 (12.5%) | 0 | 2 (8.3%) | 0 | ||||
| Fever | 1 (12.5%) | 0 | 1 (12.5%) | 1 (12.5%) | 0 (0.0%) | 0 | 2 (8.3%) | 1 (4.2%) | ||||
| Fatigue | 0 (0.0%) | 0 | 1 (12.5%) | 0 | 1 (12.5%) | 0 | 2 (8.3%) | 0 | ||||
| Nausea | 0 (0.0%) | 0 | 1 (12.5%) | 0 | 1 (12.5%) | 0 | 2 (8.3%) | 0 | ||||
| Dizziness | 0 (0.0%) | 0 | 0 (0.0%) | 0 | 2 (25.0%) | 0 | 2 (8.3%) | 0 | ||||
| Supraventricular tachycardia | 0 (0.0%) | 0 | 1 (12.5%) | 1 (12.5%) | 0 (0.0%) | 0 | 1 (4.2%) | 1 (4.2%) | ||||
Table 4: Incidence of TRAE (≥ 5%) and ≥ grade 3 TRAEs in patients receiving 12-20 mg/m2 PLM60 treatment.
A total of 12 patients had grade 3 or 4 TEAEs, among them, 12 presented with neutropenia (12/24, 50%), 10 with leucopenia (10/24, 41.7%), followed by anemia (n=1), fever (n=1) and supraventricular tachycardia (n=1). 2 (both treated with 12 mg/ m2) patients stopped treatment because of AE: one patient with hypersensitivity during the infusion of PLM60 and one with grade 3 fever. AEs leading death were reported in 3 patients, 1 in 12 mg/m2 group (unknown reason, consider unrelated with PLM60 treatment), 1 in 16 mg/m2 group (unknown reason, consider related with PLM60 treatment), 1 in 20 mg/m2 group (unknown reason, consider unrelated with PLM60 treatment).
Skin hyperpigmentation was observed in 11 patients, characterized as dark spots and blue skin, of which 10 patients were classified as grade 1, and the other 1 as grade 2. The symptom persisted for 28~144 days, and resolved with no medication. No patients discontinued PLM60 because of skin hyperpigmentation.
During the whole study period, 2 patients had abnormal ECG, including grade 1 short PR interval and grade 1 atrial tachycardia and altered T wave; cardiac arrhythmia occurred in 2 patients: one with grade 1 premature ventricular contractions (treated with 20 mg/m2); the other one patient with grade 4 supraventricular tachycardia (treated with 16 mg/m2).
Efficacy
24 patients were included in FAS for efficacy analysis. At the cutoff date of October 28, 2020, the median follow-up was 5.6 months and 2 (8.3%) patients achieved CR as best response to PLM60, 8 (33.3%) patients achieved PR, 5 (20.8%) patients classified as stable disease (SD). The change of tumor size from baseline and magnitude of response and duration after PLM60 treatment summarized in Figure 5a and 5b.
ORR of all 24 patients was 41.7% (10/24; 95% CI: 22.1~63.36), 25% (2/8), 62.5% (5/8) and 37.5% (3/8) for 12 mg/m2, 16 mg/ m2 and 20 mg/m2 group, respectively. Disease control rate (DCR) of all 24 patients was 62.5% (15/24; 95%CI: 40.59~81.20), 50% (4/8), 62.5% (5/8) and 75% (6/8) for 12 mg/m2, 16 mg/m2 and 20 mg/m2 group.
At the end of the study, PD occurred in 7 patients (n=3 for 12 mg/ m2 group, n=1 for 16 mg/m2 group, n=3 for 20 mg/m2 group) and 3 patients (n=2 for 16 mg/m2 group, n=1 for 20 mg/m2 group) died. Of 24 patients, the median PFS was 7.6 month (95% CI 3.2- NC), 9.2 months for 20 mg/m2 group more than other two dose groups (Figure 5).
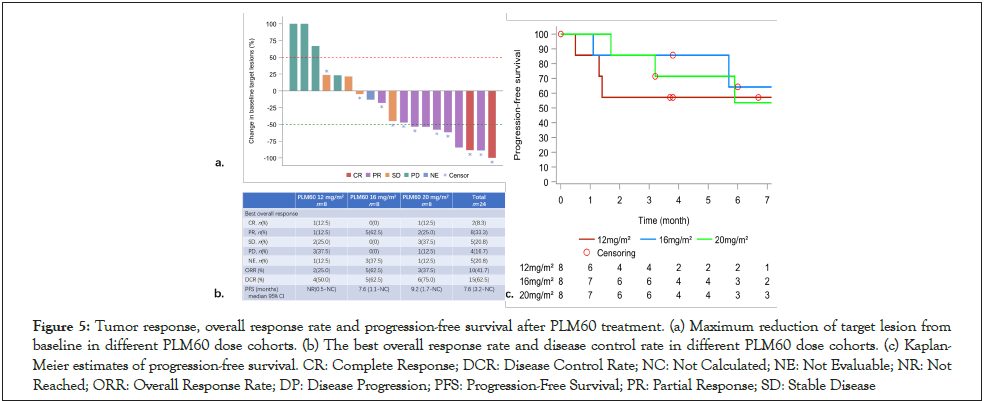
Figure 5:Tumor response, overall response rate and progression-free survival after PLM60 treatment. (a) Maximum reduction of target lesion from baseline in different PLM60 dose cohorts. (b) The best overall response rate and disease control rate in different PLM60 dose cohorts. (c) Kaplan-Meier estimates of progression-free survival. CR: Complete Response; DCR: Disease Control Rate; NC: Not Calculated; NE: Not Evaluable; NR: Not Reached; ORR: Overall Response Rate; DP: Disease Progression; PFS: Progression-Free Survival; PR: Partial Response; SD: Stable Disease
This is the first study to explore the pharmacokinetic characteristics of PLM60 in patients with relapse/refractory advanced lymphoma, and it turned out that low amount of free mitoxantrone was released in the circulation and the ratio of free mitoxantrone to total mitoxantrone was fixed with ~1% after administering PLM60 12 mg/m2-20 mg/m2.
On the basis of the result of previous phase 1 study in patients with advanced solid tumor, which showed that the PLM60’s efficacy of dose was 12 mg/m2 or more, the dosage of PLM60 in this study was determined as 12, 16 and 20 mg/m2. The pharmacokinetics result in our study was consistent with that of previous phase 1 study, which provided another evidence to support that compared with conventional mitoxantrone, PLM60 showed an altered pharmacokinetics profiles with a higher Cmax and AUC0-t (8707.14- 12848.75 μg/mL and 1024.79-1507.28 h*μg/mL) and a longer halflife time (67.69-84.00 h). Besides the long circulation characteristics, PLM60 also exhibited a limited tissue distribution in the animal experiment [10], that it preferentially accumulated into tumor zones instead of normal tissues. Accordingly, it’s the favorable pharmacokinetic characteristics of PLM60 that contribute to the reduced toxicity and improved therapeutic potential.
It’s been acknowledged that there is relationship between the accumulative dose of anthracycline and the cardiotoxicity, with the increasing accumulative dose of anthracycline, the cardiovascular damage would be irreversible [18]. Cardiotoxicities occurred by inhibiting mitochondrion and inducing energy imbalance after mitoxantrone treatment [19]. All the patients in our study have received previous treatment of anthracycline with doxorubicin accumulative dose of less than 360 mg/m2. During the whole study period, 5 patients experienced cardiovascular related AEs (one patient experienced atrial tachycardia and palpitation simultaneously), most of them were of mild intensity, and disappeared without intervention, except one patient presented with two days of intermittent palpitation, a 12-lead ECG revealed grade 4 supraventricular tachycardia, which had resolved without sequala after receiving medication for controlling the ventricular rate. No new safety signals were identified.
During the whole study, 11 patients reported skin hyperpigmentation, which was also observed in other clinical trials of PLM60 [20,21], no medical management was needed. No patients discontinued PLM60 because of skin hyperpigmentation. We assumed that its occurrence might be related with local accumulation of PLM60.
Our study demonstrated the preliminary efficacy of PLM60 among patients with relapse/refractory lymphoma. For ORR per dose group, ORR in 16 mg/m2 group and 20 mg/m2 group were higher than that of in 12 mg/m2 group, while ORR in 16 mg/m2 group was higher than that of other two dose groups with no patients achieving CR; For DCR, DCR in 20 mg/m2 group was higher than that of other two dose groups, and no significant difference was found due to the limited sample size. To be noted that, there were 6 patients with Hodgkin lymphoma enrolled, of which three in 12 mg/m2 group, two in 16 mg/m2 group and one in 20 mg/ m2 group. 4 of them was classified best response as PR, ORR was 66.7% (4/6), suggested that PLM60 might be highly effective in treatment of Hodgkin lymphoma, however, since the sample size was small, further exploration on larger population are needed.
In addition, a series of clinical trials have been conducted to examine the safety and efficacy of PLM60, we found that PLM60 monotherapy yields a promising anti-tumor activity in patients with relapsed or refractory non-Hodgkin lymphoma, advanced breast cancer and relapsed or refractory peripheral T-cell lymphoma [22,23] with a lower incidence of cardiovascular toxicity during the trials. And a Phase III trial in patients with relapsed/refractory PTCL (NCT04668690) is still ongoing.
PLM60 was designed for specifically passive targeting the tumor area, and enriching gradually in the tumor tissue, thus, it showed released steadily in the tumor tissue. Based on our study, PLM60 has an altered pharmacokinetics profiles and showed preliminary efficacy with manageable safety profiles in patients with relapse/ refractory lymphoma. Our finding suggested that PLM60 might serve as a promising treatment option in clinical practice.
We thank all the patients who participated in this study. This study was supported by CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co, Ltd. We would like to thank Lei Wang for the medical writing assistance.
Chunyan Hao, Rui Jia, Zhufeng Wu and Shaonan Ni are employees of CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd. The other authors declare no conflicts of interest.
Conception and design of the study: Yunhong Huang, Chunyan Hao
Collection of the data: Yunfei Hu, Weiwei Ouyang, Jing Zhang, Mengxiang Chen, Daoping Qing, Qiangxing Zeng and Ye Huang
Acquisition, analysis, and interpretation of the data: Chunyan Hao, Rui Jia, Zhufeng Wu, Shaonan Ni, Yunhong Huang
Writing, review, and/or revision of the manuscript: All the authors
Administrative, technical, or material support: Yunfei Hu, Weiwei Ouyang, Jing Zhang, Mengxiang Chen, Daoping Qing, Qiangxing Zeng and Ye Huang.
Citation: Huang Y, Ouyang W, Zhang J, Chen M, Qing D, Zeng Q, et al. (2023) Pharmacokinetics, Safety Profiles and Efficacy of Liposomal Mitoxantrone Hydrochloride in Patients with Relapse/Refractory Advanced Lymphoma. Chemo Open Access. 11:175
Received: 11-Jan-2022, Manuscript No. CMT-22-15616; Editor assigned: 14-Jan-2022, Pre QC No. CMT-22-15616 (PQ); Reviewed: 31-Jan-2022, QC No. CMT-22-15616; Revised: 10-Mar-2023, Manuscript No. CMT-22-15616 (R); Published: 17-Mar-2023 , DOI: 10.35248/2167-7700.23.11.175
Copyright: © 2023 Huang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.