Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Research Article - (2008) Volume 1, Issue 5
Neurodegeneration is an important component of diabetic retinopathy as demonstrated by increased neural apoptosis in the retina during experimental and human diabetes. Accumulation of sorbitol and fructose and the generation or enhancement of oxidative stress has been reported in the whole retina of diabetic animals. Aldose reductase (AR), the first and the rate limiting enzyme in the pathway reduces glucose to sorbitol and the diabetic complications are prevented by drugs that inhibit AR. In this study we examined the phosphorylation state of various retinal proteins in response to sorbitol-treatment by phospho-site-specific antibody microarray. Our results suggest that various retinal protein kinases and cytoskeletal proteins either activated or down regulated in response to sorbitol treatment. Further, our study also indicates the activation of retinal insulin and insulin growth factor 1 receptor and their downstream signaling proteins such as phosphoinositide 3-kinanse and protein kinase B (Akt). Understanding the regulation of retinal proteins involved in polyol (sorbitol) pathway would help to design therapeutic agents for the treatment of diabetic retinopathy.
Keywords: Diabetes, Insulin receptor signaling, Phosphoinositide 3-kinase, Protein kinase B, Protein phosphorylation, Cytoskeletal proteins, Insulin growth factor-1 receptor.
The polyol pathway of glucose metabolism is active when the intercellular glucose levels are elevated in the cell (Gabbay, 1973). Aldose reductase (AR), the first and the rate limiting enzyme in the pathway reduces glucose to sorbitol using NADPH as a cofactor (Lorenzi, 2007). Sorbitol is then metabolized to fructose by sorbitol dehydrogenase (SDH) that used NAD+ as cofactor (Lorenzi, 2007). Sorbitol is an alcohol that is polyhydroxylated, and strongly hydrophilic and does not diffuse readily through cell membranes and accumulates intracellularly with possible osmotic consequences (Gabbay, 1973). The fructose produced by the polyol pathway can get phosphorylated to fructose 3-phosphate (Szwergold et al., 1990), which can be further broken down to 3-deoxyglucosone, and both these compounds can be very powerful glycosylating agents that can result in the formation of advanced glycation end products (AGEs) (Szwergold et al., 1990). Thus activation of polyol pathway, by altering the intracellular homeostasis, generating AGEs, and exposing cells to oxidant stress due to decreased antioxidant defense mechanism and generation of oxidant species can initiate several mechanisms of cellular damage.
Accumulation of sorbitol and fructose and the generation or enhancement of oxidative stress has been reported in the whole retina of diabetic animals (Gabbay, 1975; Dagher et al., 2004; Lorenzi, 2007). The retinas of experimentally derived diabetic rats show increased lipid peroxidation (Obrosova et al., 2003), increased nitrotyrosine formation (Obrosova et al., 2005) and depletion of antioxidant enzymes (Obrosova et al., 2003). These abnormalities are prevented by drugs that inhibit AR (Dahlin et al., 1987; Tomlinson et al., 1992; Narayanan, 1993; Tomlinson et al., 1994; Obrosova et al., 2003; Lorenzi, 2007). Retinas from diabetic patients with retinopathy show more abundant AR immunoreactivity in ganglion cells, nerve fibers, and Muller cells than retinas from non-diabetic individuals (Vinores et al., 1988). It has also been shown that human retinas from non-diabetic eye donors exposed to high glucose levels in organ cultures accumulate sorbitol to the same extent as similarly incubated retinas of non-diabetic rats (Dagher et al., 2004). Retinal ganglion cells, Muller glia, vascular pericytes and endothelial cells are endowed with AR in all species (Dagher et al., 2004) and these cells are known to be damaged in diabetes (Lorenzi and Gerhardinger, 2001). The retinal vessels of diabetic rats treated with sorbinill, an AR inhibitor for the 9 months duration of diabetes, showed prevention of early complement activation, decreased levels of complement inhibitors, microvascular cell apoptosis and acellular capillaries (Dagher et al., 2004). Based on the data from the animal models, there is evidence for the concept that polyol pathway activation is a sufficient mechanism for the retinal abnormalities induced by diabetes in rats.
In the present study we examined the retinal IR and insulin growth factor-1 receptor (IGF-1R) signaling in sorbitol-treated retinas ex vivo and show that sorbitol activates both the IR and IGF-1R tyrosine kinases, which results in activation of the receptor’s direct downstream targets. This receptor activation leads to the activation of PI3K and Akt survival pathway in the retina. With the advent of phospho-site-specific antibody microarray, we observed that sorbitol-treated retinas exhibit either increased or decreased phosphorylation of several tyrosine, serine/threonine kinases and cytoskeletal proteins which are downstream effector molecules of IR and IGF-1R signaling pathways.
Materials—Polyclonal anti-IRß, anti-IGF-1R and monoclonal anti-PY-99 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The actin antibody was obtained from Affinity BioReagents (Golden, CO). Sorbitol was obtained from Sigma (St Louis, MO). Anti-pAkt (S473) and anti-Akt antibodies were obtained from Cell Signaling (Beverly, MA). All other reagents were of analytical grade and from Sigma (St. Louis, MO).
Animals-All animal work was done in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology on the Use of Animals in Vision Research. All protocols were approved by the IACUC at the University of Oklahoma Health Sciences Center and the Dean McGee Eye Institute. In all experiments, rats were killed by asphyxiation with carbon dioxide before the retinas were removed.
Retinal organ cultures—Retinal organ cultures were carried out as previously described (Rajala et al., 2004; Rajala et al., 2007). Retinas were removed from Sprague-Dawley albino rats that were born and raised in dim cyclic light (5 lux; 12 h ON: 12 h OFF), and incubated for 30 min at 37 °C in Dulbecco’s modified Eagle’s (DMEM) medium (Gibco BRL) in the presence of sorbitol. Control cultures were carried out in the absence of additives. At the indicated times, retinas were snap-frozen in liquid nitrogen and stored at – 80 °C until analyzed. The retinas were lysed in lysis buffer [1% NP 40, 20 mM HEPES (pH 7.4), and 2 mM EDTA] containing phosphatase inhibitors (100 mM NaF, 10 mM Na4P2O7, 1 mM NaVO3 and 1 mM molybdate) and protease inhibitors (10 μM leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF), and kept on ice for 10 min followed by centrifugation at 4 ºC for 20 min.
PI3-kinase assay—Enzyme assays were carried out as previously described (Rajala et al., 2007). Briefly, assays were performed directly on IRß immunoprecipitates of retinal lysates prepared from sorbitol treated or untreated lysates in 50 μl of reaction mixture containing 0.2 mg/ml PI- 4,5-P2, 50 μM ATP, 10 μCi [ 32P]ATP, 5 mM MgCl2, and 10 mM HEPES buffer (pH 7.5). The reactions were carried out for 30 min at room temperature and stopped by the addition of 100 μl of 1 N HCl followed by 200 μl of chloroform/ methanol (1/1, v/v). Lipids were extracted and resolved on oxalate-coated TLC plates (silica gel 60) with a solvent system of 2-propanol/2M acetic acid (65/35, v/v). The plates were coated in 1% (w/v) potassium oxalate in 50% (v/v) methanol and then baked in an oven at 100 °C for 1 hr prior to use. TLC plates were exposed to X-ray film overnight at –70 °C and radioactive lipids were scraped and quantified by liquid scintillation counting.
Immunoprecipitation—Retinal lysates were prepared as previously described (Li et al., 2007; Rajala et al., 2007). Insoluble material was removed by centrifugation at 17,000 x g for 20 min at 4 °C, and the solubilized proteins were precleared by incubation with 40 ml of protein A-Sepharose for 1 h at 4 °C with mixing. The supernatant was incubated with primary antibodies overnight at 4 °C and subsequently with 40 ml of protein A-Sepharose for 2 h at 4 °C. Following centrifugation at 17,000 x g for 1 min at 4 °C, immune complexes were washed three times with ice-cold wash buffer [50 mM HEPES (pH 7.4) 118 mM NaCl, 100 mM NaF, 2 mM NaVO3, 0.1% (w/v) SDS and 1% (v/v) Triton X-100]. The immunoprecipitates were either subjected to Western blotting analysis or used to measure the PI3K activity.
SDS-PAGE and Western blotting-Proteins were resolved by 10% SDS-PAGE and transferred onto nitrocellulose membranes. The blots were washed twice for 10 min with TTBS [20 mM Tris-HCl (pH 7.4), 100 mM NaCl, and 0.1% Tween-20] and blocked with either 5% bovine serum albumin or non-fat dry milk powder (Bio-Rad) in TTBS for 1 h at room temperature. Blots were then incubated with anti-PY99 (1:1000) or anti-pAkt (1:1000) or anti-Akt (1:1000) or anti-IRß or anti-IGF 1R (1:1000) or anti-actin (1:1000) antibodies overnight at 4 °C. Following primary antibody incubations, immunoblots were incubated with HRP-linked secondary antibodies (either anti-rabbit or anti-mouse) and developed by ECL according to the manufacturer's instructions.
Phospho-site-specific antibody microarray-Sorbitoltreated and untreated retinas were homogenized in ice cold lysis buffer [1% NP 40, 20 mM HEPES (pH 7.4), and 2 mM EDTA] containing phosphatase inhibitors (100 mM NaF, 10 mM Na4P2O7, 1 mM NaVO3 and 1 mM molybdate), protease inhibitors (10 μM leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF) and disulphide reducing agent (1 mM dithiothreitol). The final protein concentration in SDS-PAGE sample buffer was adjusted to 1mg/ml. The samples were sent to Kinexus Bioinformatics Corporation (Vancouver, British Columbia, Canada) for pan-specific and phosphosite- specific antibody microarray analysis. The Kinexus utilize antibody microarrays to track the differential binding of dye-labeled proteins in lysates prepared from retinal tissues. The results can provide productive insights into differences in protein expression and phosphorylation status in control and sorbitol-treated conditions.
Sorbitol-induced tyrosine phosphorylation of several retinal proteins—Retinal proteins were treated with varying concentrations of sorbitol (0-3.0 M) and subjected to Western blot analysis with the anti-PY99 antibody. The results indicated a significantly increased level of tyrosine phosphorylation in the retinal proteins in the 1.0 and 2.0 M sorbitol treatment compared to the untreated retinas (Fig. 1A). We observed the tyrosine phosphorylation of several retinal proteins, with apparent molecular weights of 170, 130, 115, 79, 70 and 41 kDa. The blot was stripped and reprobed with actin (Fig. 1B) to ensure that equal amounts of protein were loaded. These results suggested that under our experimental conditions, sorbitol induced the tyrosine phosphorylation of several retinal proteins.
Figure 1: Sorbitol-induced tyrosine phosphorylation of several retinal proteins. Retinas were cultured in DMEM in the presence or the absence various concentrations of sorbitol for 30 min at 37 °C. Thirty micrograms of retinal proteins were subjected to Western blot analysis with anti- PY 99 antibody (A). The blot was reprobed with the antiactin antibody to ensure equal amount of protein in each lane (B). All experiments were carried out in duplicate.
Sorbitol-induced activation of insulin- and insulin-like growth factor-1 receptor—To determine the sorbitol-induced activation of IR and IGF-1 receptors, we immunoprecipitated retinal lysates from control and sorbitol-treated organotypic cultures with anti-IRß (Fig. 2B) and anti-IGF1R (Fig. 2D) antibodies followed by Western blot analysis with anti-PY99 antibody. The results indicated the activation of IR (Fig. 2A and IGF 1R (Fig. 2C) in response to sorbitol-treatment.
Figure 2: Sorbitol-induced activation of IR and IGF-1R. Retinal proteins from control and 1.0 M sorbitol-treated organotypic cultures were immunoprecipitated with anti- IRß (B) or anti-IGF-1R (D) antibody followed by Western blot analysis with anti-PY99 antibody (A and C). The blots were stripped and reprobed with anti-IRß antibody or anti-IGF-1R antibody to ensure equal amounts of IR and IGF-1R in each immunoprecipitate. Sorbitol-induced activation IR associated PI3K activity. Retinas were cultured in DMEM and treated with various concentrations of sorbitol for 30 min at 37 °C. TLC autoradiogram of PI3K activity measured in anti-IRb immunoprecipitates of retinas using PI-4,5-P2 and [g32P]ATP as substrates. The radioactive spots of PI-3,4,5-P3 were scraped from the TLC plate and counted (E). Sorbitol-induced activation of Akt. Sorbitol treated and untreated retinal proteins were subjected to Western blot analysis with anti-pAkt (S473) (F) and anti-Akt (G) antibodies. All experiments were carried out in duplicate.
Sorbitol induced activation of IR associated PI3K activity—We have previously reported the activation of PI3K through tyrosine phosphorylated IR in the retina (Rajala et al., 2007). To determine whether the activation of PI3K is regulated through IR, we have immunoprecipitated IR from retinal lysates that were prepared from non stimulated control and sorbitol-treated organotypic cultures, and measured the PI3K activity. The results indicated an increased PI3K activity with IR from sorbitol-treated retinas (Fig. 2E). These results suggested that sorbitol-induced activation of PI3K occurs via activation of the IR.
Phospho-site-specific antibody microarray-To determine the global changes in the phosphorylation (tyrosine and serine/threonine) of retinal proteins in response to sorbitoltreatment , we examined the phosphorylation state of retinal proteins by antibody microarray. Of 273 phospho-sitespecific antibodies 32 proteins were found to exhibit either increased or decreased phosphorylation (Table 1). These proteins include serine/threonine and tyrosine kinases and mainly proteins involved in the cytoskeletal organization. These results suggest that sorbitiol-treatment induces the activation of several protein kinases which may in turn regulate the cytoskeletal reorganization.
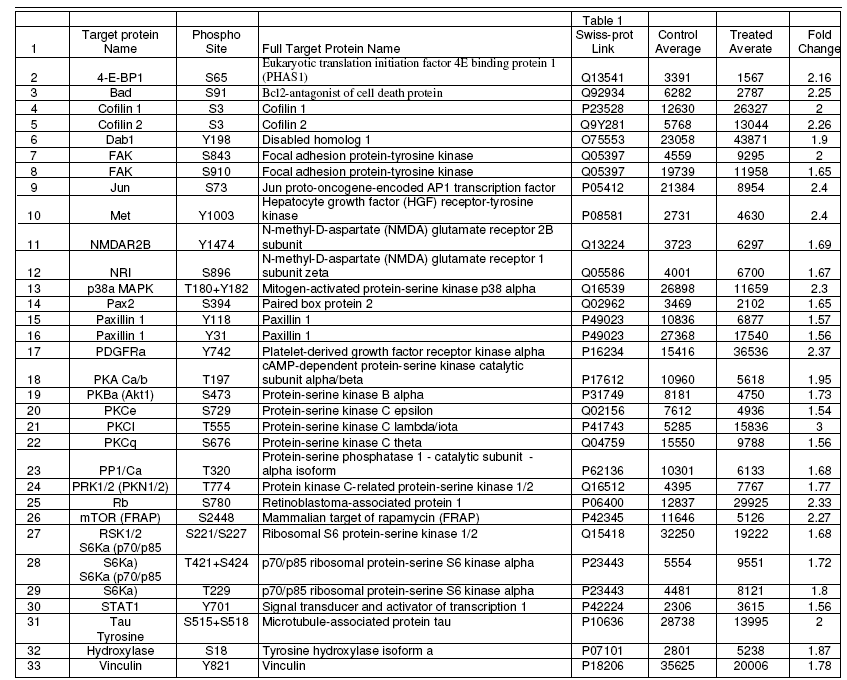
Table 1: Phosphorylation status of various retinal protein kinases and cytoskeletal proteins.
The sorbitol pathway, non-enzymatic glycation of proteins and increased oxidative stress are known to activate protein kinase C which is an effective activator of MAPKs (Tomlinson, 1999). These kinases phosphorylate transcription factors, which in turn alter the balance of gene expression and promote the development of diabetic nephropathy, retinopathy and neuropathy (Tomlinson, 1999). The normal retinal IR exhibits high constitutive activity that is reduced in diabetes (Reiter et al., 2006). The diabetic rat retina further shows loss of PI3K, Akt1 and Akt-3, mTOR and p70S6K activities and increased GSK3ß activity (Reiter and Gardner, 2003). Elevated levels of sorbitol have been shown to be implicated in the pathogenesis of diabetic retinopathy (Mizutani et al., 1998; Lorenzi and Gerhardinger, 2001; Asnaghi et al., 2003; Dagher et al., 2004; Lorenzi, 2007). The rate limiting step in the pathway, aldose reductase which reduces the glucose to sorbitol is the major therapeutic target for diabetic retinopathy (Dvornik et al., 1973; Kinoshita et al., 1979; Dahlin et al., 1987; Tomlinson et al., 1992; Chandra et al., 2002; Obrosova et al., 2003; Obrosova et al., 2005; Lorenzi, 2007). In this study, like insulin, sorbitol was found to induce tyrosine phosphorylation of IR and IGF- 1R. It was reported previously that insertion of IR into the plasma membrane is necessary for sorbitol-induced IR activation (Ouwens et al., 2001). Consistent with these observation we reported that IRs in rod outer segments of retinas are localized to plasma membrane (Rajala et al., 2007). Further studies, however, are required to understand how the IR kinase activity becomes reduced in diabetes.
In the present study, in response to sorbitol, we have observed increased activation of PI3K through IR activation. In some neuronal cell types, such as cerebellar granular neurons (59) and PC-12 cells (60), receptor activation of PI3K has been shown to protect these cells from stressinduced neurodegeneration. Further, IR activation has been shown to rescue retinal neurons from apoptosis through a phosphoinositide 3-kinase (PI3K) cascade (Barber et al., 2001; Barber et al., 1998). We have previously reported that under physiological conditions, light-induced the tyrosine phosphorylation of retinal IR which leads to the activation of PI3K (Rajala et al., 2002). The earlier studies along with the results from the present study clearly suggests that sorbitol also induces the activation of PI3K associated with the tyrosine phosphorylated IR. In this study we also observed the activation of IGF-1R. The precise molecular mechanism of IR and IGF-1R activation is not known. It has been shown that the non-receptor tyrosine kinase Src phosphorylates insulin- and insulin-like growth factor receptors on autophosphorylation sites (Yu et al., 1985; Peterson et al., 1996). Thus, the Src kinase has been shown to substitute for the ligand-dependent receptor activation (Peterson et al., 1996; Yu et al., 1985). Furthermore, the IGF-1 receptors in ROS are localized to plasma membrane (Dilly and Rajala, 2008) and it was reported previously that insertion of the IR into the plasma membrane is necessary for hyperosmotic stress-induced receptor activation (Ouwens et al., 2001). Such possibility can not be rule be ruled out for IGF-1R activation. In recent years it has become apparent that receptor tyrosine kinases (RTKs) and the signaling pathways they activate are part of a large signaling network that can be regulated by multiple extracellular cues such as cell adhesion, agonists of G protein coupled receptors, lymphokines or stress signals (Carpenter, 1999). RTKs have also been shown to be activated by membrane depolarization by various stress responses including hyperosmotic conditions, ultraviolet radiation and white light as well as by G protein coupled receptors (Brown and Cornwall, 1975). Consistent with these studies, we recently reported that the state of IR phosphorylation is regulated through the photobleaching of G-protein coupled receptor rhodopsin (Rajala et al., 2007). Photoreceptor cell membranes are more susceptible to lightinduced depolarization (Brown and Cornwall, 1975). These possibilities can not be ruled out in the sorbitol-induced activation of IGF-1 receptors.
Neurodegeneration is an important component of diabetic retinopathy as demonstrated by increased neural apoptosis in the retina during experimental and human diabetes (Barber et al., 1998). IR activation has been shown to rescue retinal neurons from apoptosis through a phosphoinositide 3-kinase and protein kinase B (Akt) survival cascade. A significant decrease of retinal IR kinase activity has been reported after 4 weeks of hyperglycemia in STZ treated rats (Reiter et al., 2006). Sorbitol-induced hyperosmotic-stress responses interact with the insulin signaling pathways at several levels (Ouwens et al., 2001). Sorbitol has been previously shown to induce the tyrosine phosphorylation of IR (Ouwens et al., 2001).
The ability of osmotic shock to directly stimulate tyrosine phosphorylation events was confirmed by phosphotyrosine immunoblotting. Several discrete tyrosine-phosphorylated proteins in the range of 115-170 kDa and 41-79 kDa were clearly induced by osmotic shock treatment. Previous studies have also reported the activation of tyrosine phosphorylation in response to hyperosmotic stress (Chen et al., 1997; Hresko and Mueckler, 2000; Janez et al., 2000). The Kinexus antibody microarray suggest the changes in the phosphorylation status of several cytoskeletal proteins (Cofillin 1 and 2, Paxillin 1, Vinculin) and several protein kinases (Focal adhesion protein-tyrosine kinase, Hepatocyte growth factor receptor-tyrosine kinase, Mitogen- activated protein-serine kinase p38alpha, Platelet-derived growth factor receptor kinase alpha, cAMP-dependent protein-serine kinase catalytic subunit alpha.beta, Protein- serine kinase B alpha, Protein-serine kinase C epsilon, Protein-serine kinase C lambda/iota, Protein-serine kinase C theta, Protein kinase C-related protein-serine kinase 1/2 Ribosomal S6 protein-serine kinase 1/2 , and p70/p85 ribosomal protein-serine S6 kinase alpha).
Cytoskeletal boundary protein and the plasma membrane control cell shape, delimit specialized membrane domains and stabilize attachments to other cells and to the substrate (Luna and Hitt, 1992; Sechi and Wehland, 2000). The IRs in the retina, especially in the rod outer segments, are localized to plasma membrane (Rajala et al., 2007). This suggests that IR is in close association with the cytoskeleton. It is not clear how the high extracellular glucose effects inside retinal cells during hyperglycemia. However, it has previously been shown that high glucose alters the physical properties of extracellular matrix through the non-enzymatic glycation of proteins, leading to changes in the organization of the intracellular actin cytoskeleton (Howard et al., 1996). Additionally, dysfunction of the actin cytoskeleton is a key event in the pathogenesis of diabetic nephropathy (Conway et al., 2004), diabetic neuropathy (McLean, 1997; McLean et al., 1995) and diabetic cardiomyopathy (Olson et al., 1998; Fein et al., 1984). Further studies are required to understand the role of regulation of cytoskeletal proteins in diabetic retinopathy.
IR, Insulin Receptor; PI3K, Phosphoinositide 3-kinase; IRß, IR Beta subunit; IGF 1R, Insulin-like growth factor-1 receptor; SDS-PAGE, Sodium dodecyl sulfate polyacrylamide gel electrophoresis; IPs, Immunoprecipitates.
The project described was supported by grants from the National Eye Institute (R01EY016507). The content is solely the responsibility of the author and do not necessarily represent the official views of the National Eye Institute, or the National Institutes of Health.