Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2012) Volume 3, Issue 5
The photocatalytic oxidation of crystal violet, a triphenyl methane dye in aqueous solutions was investigated with nanoanatase TiO2 containing Anatase and rutile phases in the ratio of 3:1, under UV light by using a 125 W high pressure mercury vapor lamp as the source. The dye degradation using Ag+ doped TiO2 and nanoanatase TiO2 was compared. An optimum catalyst dose of 1 g/L was used. It was found that nanoanatase TiO2 had a higher efficiency than the Ag+ doped Titanium di Oxide. Nanoanatase TiO2 was found to be easy to separate from the treated effluent by simple centrifuging. The degradation of the dye of initial concentration: 5×10-5 mol/L, using nanoanatase TiO2 was greater than 99.5% on UV illumination for 45 minutes and that with Ag+ doped Titanium di Oxide as catalyst, was found to be 75% for 45 minutes of illumination. The effects of various parameters such as pH; initial dye concentration and catalyst dose on the reaction rate were studied. The kinetics of degradation fit well to Langmuir- Hinshelwood rate law.
Keywords: Crystal violet; Photocatalysis; Anatase
Photolysis is the process of decomposition in the presence of a form of light, whereas photocatalysis is defined as the acceleration of a photochemical reaction in the presence of a catalyst. Catalysts are the substances which are used in order to provide an alternative path for a reaction to take place, which is done in order to enhance the rate of a reaction. Now the removal of the color from the waste water discharge from various industries such as the textile, paper, plastic, leather, ink etc. is an active issue all over the world. Use of dyes in these industries further asserts the issue at hand. There are many classes of dyes such as acidic, basic, neutral, azo, direct, reactive etc. Azo dyes are the most frequently used; these contain one or more azo bonds in their structure (-N=N-). These dyes can be toxic and potentially carcinogenic [1-5].
There have been many ways to treat the dye effluents, viz. biological methods, flocculation, reverse osmosis, adsorption on activated charcoal, chemical oxidation and advanced oxidation processes (AOPs). AOPs have been proven to be the most efficient way to degrade the dye effluents. The term AOP is often used when the oxidative capacity of the parent oxidant is enhanced to make the oxidation process more efficient. In water treatment, AOPs are generally a combination of processes involving O3, H2O2, and UV light [6]. Photocatalysis using TiO2 is one of the most efficient methods to degrade dyes/pollutants. Crystal violet or Gentian violet is a triarylmethane dye (Figure 1).
Crystal violet has been used for various purposes; it is extensively used in textile dying and paper coloring. In the present work the photocatalytic degradation of this dye will be carried out using the nanoanatase TiO2 catalyst in the photo reactor. Effects of various factors such as concentration of the dye, dose of photocatalyst, presence of light etc. have been studied [7].
Crystal violet dye was purchased from Merck India. Millipore water was used as the solvent for all the studies. A 125 W high pressure mercury vapor lamp was purchased from Samson India and was used as the light source.
Preparation of photocatalyst
The nanoanatase TiO2 contains anatase and rutile phases in a ratio of about 3:1. Transmission electron microscopy showed that the anatase and rutile particles separately form their agglomerates. The average sizes of the anatase and rutile elementary particles are 85 and 25 nm, respectively. Diffuse reflectance spectra of the TiO2 powder were successfully traced by physically mixing pure anatase and rutile particles in a ratio of 3:1. By the HF treatment of the TiO2 powder, pure rutile particles were isolated [8]. All these results indicate that the rutile phase does not exist as an over layer on the surface of anatase particles, but it exists separately from anatase particles. The X-ray diffraction (XRD) patterns of catalysts were recorded on a Siemens (D-5005) diffractometer with a scan rate of 2 min-1. JEOL JEM-200 CX operated at 200 kV was used to carry out Transmission Electron Microscopy (TEM) studies [9-11]. We also found that photocatalytic oxidation of naphthalene is inefficient on pure anatase and rutile powders. However, the reaction is very efficient on the P-25 powder, as well as on a mixture of pure anatase and rutile particles. Under the conditions of the photocatalytic reactions, the anatase and rutile agglomerates are considered to be decomposed, and the anatase and rutile particles are in contact, leading to a synergy effect.
Instrumentation
The instruments used for the study were PG Instruments T60 UV-Vis Scanning Spectrophotometer, 125 W high pressure mercury vapor lamp (Samson India), General Purpose Laboratory Centrifuge (Laboratory Centrifuge) Remi R-40 and Remi magnetic stirrer [12].
The experimental setup consists of a quartz tube along with a jacket with dimensions of 3.4 cm inner diameter, 4 cm outer diameter and 21 cm length and a glass reactor of 7.2 cm length and 5.2 cm inner diameter. The outer glass shell is removed and placed inside the quartz tube for use. The ballast and capacitor were connected in series with the lamp to avoid fluctuations in the input power supply. Submersible water pump was used for circulating water through the jacket of the quartz tube to avoid heating due to dissipative loss of UV light.
Adsorption and direct photolysis
The adsorption studies were conducted in the dark conditions using 5×10-5 dye solution (100 ml) with 1 g/L nanoanatase TiO2 catalyst. Aliquots were taken at regular time interval and centrifuged. The dye concentration was measured spectrophotometrically. Direct photolysis of the dye was also studied in the absence of the photocatalyst.
Photocatalytic degradation
A 5×10-5 mol/L stock solution was prepared with millipore water from which an experimental solution was derived of 2.5×10-5 mol/L concentration. The experimental setup as shown in figure 2 consists of a 100 ml beaker placed upon a magnetic stirrer on top of which a 125 W high pressure mercury lamp emitting UV light of wavelength ∼ 365 nm was placed [13-15]. This served as the light source for the Photocatalysis. 40 ml of the working solution was taken in the beaker and was added nanoanatase TiO2 to it. Catalyst dose of 1 g/L was added to the dye solution. The height of the solution in the beaker was 4 cm. The solution was stirred in the dark for about 30 min to establish adsorption equilibrium, the zero time reading taken after the equilibrium was reached and the solution was irradiated. Aliquots were taken at regular time intervals and were analyzed using the spectrophotometer. The percentage degradation and the percentage decolouration were calculated [16,17].
Calibration
The absorbance of Crystal violet at various dye concentrations was measured. Absorbance vs. Concentration was plotted and it was found to be a linear curve as shown in figure 3.
Catalyst characterization
The catalyst nanoanatase TiO2 was characterized by a scanning electron microscope. Scanning electron microscopy affirmed that the anatase and rutile particles separately form their agglomerates. The particle size for nanoanatase TiO2 was found to be 19 nm as shown in figures 4a and 4b. The SEM micrographs of TiO2 doped with 2% Ag+ ions were also analyzed and found to be 200 nm in size. Ag-doping can induce electron-hole separation and as an electrical field aiding electron excitation. Normally, Ag atoms deposit on the surface of TiO2 or form a core-shell structure such as Ag-TiO2. XRD patterns of pure TiO2 and Ag+ TiO2 are shown in figures 4c and 4d respectively. The pattern can be indexed to TiO2 in anatase phase only. It may be noted that the background of the X-ray pattern is flat indicating that TiO2 is crystalline. The lattice parameter for pure nanoanatase TiO2 is a=3.7865 Å and c=9.5091 Å. There was no significant change in the lattice parameters in case of Ag+ doped TiO2.
The crystallite size is determined from XRD pattern using Sherrer formula.
t=0.9λ/β cosθ. Here “t” is in nm, λ is the wavelength of X-ray in Å, β is FWHM in radians and θ is the Bragg angle.
Transmission electron micrographs of TiO2 show fine crystals with the mean size of 18 ± 2 nm which agrees well with the XRD measurements. Typical TEM image of TiO2 is shown in figure 4e.
Photocatalytic degradation
The maximum wavelength observed for the crystal violet was 583 nm and the degradation of the crystal violet solution of 2.5×10-5 mol/L was observed to be >99% and the degradation for a concentration of 5×10-5 mol/L was found to be >99.9%. These results were obtained after an irradiation time of about 25 min and 45 min respectively, as shown in the figure 5.
When a photon of UV light strikes the surface of TiO2 a valance bond electron moves into the conduction band thus forming a positively charged hole in the valence band. The conduction band electron and the valence band holes then migrate to the oxide surface and react with the chemisorbed O2 or OH-/H2O molecules to generate reactive oxygen species such as O2-, HOO, OH- radicals, which attack the dye molecules and the degradation commences. Alternatively, the electron in the conduction band can be picked up by the adsorbed dye molecules, leading the formation of dye radical anion. When the photocatalyst titanium-di-oxide absorbs ultraviolet radiation from sunlight or illuminated light source (flouroscent lamps), it will produce pairs of electrons and holes. The electron of the valence band of titanium-di-oxide becomes excited when illuminated by light. The excess energy of this excited electron promotes the electron from the valence band to the conduction band of titanium-di-oxide, therefore creating the the negative electron (e-) and positive hole (h+) pair. This stage is referred to as semiconductor’s photo excitation state. The energy difference between the valence band and conduction band is known as band gap.
Wavelength of light necessary for photoexcitation is:
= Planck’s constant / Band gap (eV)
= 1240 / 3.2
= 388 nm.
The positive hole of TiO2 breaks apart the water molecule from the moisture in the air and forms hydrogen H2 gas and hydroxyl radical (OH-). The negative electron reacts with O2 molecue to from super oxide anion. This cycle continues as long as light is available. Alternatively, the electron in the conduction band can be picked up by the adsorbed dye molecules, leading to the formation of dye radical anion and subsequent reaction of the dye radical anion can lead to the degradation of the dye. Also the adsorbed dye molecules may be directly oxidised by the valence band holes to for dye radical cations which ultimately cause dye degradation [12].
Adsorption and direct photolysis
The results of adsorption studies of dye solution (5×10-5 mol/L) with nanoanatase TiO2 (1 g/L), are shown in the figure 6 nanoanatase TiO2 showed 25% adsorption after 120 min. Direct photolysis of the dye solution by constant stirring and irraditiation in the absence of a photocatalyst showed no considerable degradation even after 2 hours (Table 1).
| Time | % Adsorption with nanoanatase TiO2 | % Adsorption with Ag+ doped TiO2 |
|---|---|---|
| 0 | 0 | 0 |
| 15 | 14.369 | 10.73 |
| 30 | 22.3 | 13.14 |
| 45 | 25.62 | 23.9 |
| 60 | 27.34 | 25.12 |
| 90 | 28.17 | 25.19 |
| 120 | 28.92 | 25.14 |
Table 1: Adsorption studies of dye solution with nanoanatase.
Kinetic analysis
The Langmuir-Hinshelwood kinetic model illustrates the dependence of the rate of dye degradation on the concentration of the dye, which is given by
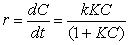
In this equation KC is almost 1, hence we can neglect KC which gives us the equation which can be simplified into a pseudo first order equation
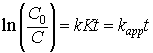
Here, r is the rate of degradation (mol/L/min), C0 is the initial concentration of the dye solution (mol/L/min) and C is the concentration of the dye solution at a time t (mol/L/min), t is the time of irradiation (min), and K the adsorption coefficient of the dye onto the photocatalyst particles (L/mg). The graphs obtained in the figure 7 were found to be in the pseudo first order as confirmed by the linear transform in the ln (C0/C)=kappt. The rate constants were obtained as follows,
At 5×10-5 mol/L: kapp=0.1237, R2=0.9269
At 2.5×10-5 mol/L: kapp=0.2022, R2=0.9655
Effect of initial dye concentration
The effect of dye concentration on its degradation was studied. Figure 8 shows the percentage degradation at various initial dye concentrations. At higher concentration, the degradation was found to be less. This may be because as the concentration of the dye increases, the catalyst particles adsorb more and more dye. Hence, the ultraviolet (UV) light does not reach the catalyst surface. At higher concentration, the light travels up to a smaller distance.
Effect of catalytic dose
The dye solution (20 ppm) was irradiated for 120 min with different catalytic doses (0.1 g/L, 0.4 g/L, 0.6 g/L, 1 g/L, 1.1 g/L, 1.2 g/L). Figure 9 shows a plot of percentage degradation of dye vs. catalytic dose. The degradation increases with increase in catalytic dose. At 0.8 g/L catalytic dye was found to be almost completely decolourised on irradiation for 120 minutes. Hence, an optimum catalyst dose of 1 g/L was used.
Effect of pH
Studies were conducted on the effect of pH on the dye degradation. This was done because the effluents of a textile industry are usually at different pH. It was observed that the pH decreased on the photocatalytic degradation. The dye degradation was conducted on a range of pH between 3 and 13 after irradiation for 120 minutes. The initial pH was adjusted by 1 N HNO3. It was observed that the dye degradation varied only within 2% by pH change.
Chemical actinometry
To measure the intensity of the radiation from the lamp, we plotted a graph of average concentration of NBA versus the time and found out the slope of the curve. The plot is as shown below (Figure 10).
The best fit was a straight line. The slope of the line at t=0 was found to be:
Slope=-1.700
At R2=0.919
The value of the flux for 125 W a high pressure mercury vapour lamp was found to be 3.464 Wm-2 (Table 2).
| Parameters | Values | Units |
|---|---|---|
| φ | 0.5 | |
| K0 | 2.83333E-07 | (mol/L/sec) |
| I0 = K0/φ | 5.66667E-07 | (einstein/L/s) |
| F0 = (I0 V NA Eλ ) / A | ||
| V | 0.05 | L |
| NA | 6.023E+23 | |
| Eλ = hc/λ | J / Photon | |
| h | 6.63E-34 | J s / Photon |
| λ | 0.000000365 | m |
| c | 300000000 | m / s |
| Eλ | 5.44932E-19 | J / Photon |
| A = 2πdl /2 | 0.0026847 | m2 |
| F0 = (I0 V NA Eλ) / A | 3.463830963 | W/m2 |
Table 2: Parameter values and its units.
The ultraviolet (UV) light irradiation of the dye by using nanoanatase TiO2 as a catalyst has yielded percentage decolouration of greater than 99% for a catalyst loading of 1 g/L and initial concentration of the dye solution of 5×10-5 and 2.5×10-5 mol/L. The rate constant of degradation was found to be 0.1237 and 0.2022 respectively. This can be an efficient and cost effective method of treating this azo-dye [17].