Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2023)Volume 11, Issue 3
Sudden wilt syndrome of chilli pepper is an emerging disorder characterized by unexpected and abrupt wilting of plants due to water stagnation and resultant hypoxic conditions in the rhizosphere. This problem is further exacerbated by infection of soilborne fungus Fusarium oxysporum, which is not the primary cause. The study is an attempt to investigate physiological and biochemical alterations in three test organs (roots, leaves and fruits) of chilli pepper in response to sudden wilt syndrome. The sudden wilt-affected plants had lower levels of total phenols, o-dihydroxyphenols, flavonoids, ascorbic acid, peroxidase, polyphenol oxidase, catalase, phenylalanine ammonia lyase, tyrosine ammonia lyase, polyphenol oxidase, lignin, moisture content in leaves, Relative Water Content (RWC), Membrane Stability Index (MSI), chlorophyll A and B and the gas exchange parameters (net photosynthesis, transpiration rate and stomatal conductance), all indicating towards an impaired defense system. On the contrary, there was a rise in the contents of hydrogen peroxide and malondialdehyde as well as proline, suggesting the onset of adaptive mechanisms that maintain normal osmoregulation in the stressed plants. The contents of total sugars and proteins were not affected in diseased plants in comparison to the healthy plants. As the first systematic approach to understanding sudden chilli wilting, this study explains the biochemical and physiological changes that lead to this phenomenon.
Abiotic stress; Plant defense; Oxidative stress; Polypropanoid pathway; Relative water content; Net photosynthesis
ROS: Reactive Oxygen Species; CAT: Catalase; POX: Peroxidase; AOX: Antioxidants; PPO: Polyphenol Oxidase; RWC: Relative Water Content; MSI: Membrane Stability Index; A: Net Photosynthetic rate; Gs: Stomatal conductance; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; PAL: Phenylalanine Ammonia Lyase; TAL: Tyrosine Ammonia Lyase; MCL: Moisture Content in Leaves; Chl: Chlorophyll; E: Transpiration rate; WUE: Water Use Efficiency
Chilli pepper (Capsicum annuum L.) has captivated imaginations and taste buds of mankind for thousands of years, both as spice and as vegetable. More than one-quarter of the world’s population consumes chilli peppers on a daily basis and have been increasingly admired as an integral component of numerous world cuisines [1]. Chilli is susceptible to several parasitic diseases caused by more than 50 eukaryotic, prokaryotic and viral pathogens as well as non-parasitic disorders related to nutrients, chemicals and the weather. Chilli growers in India have been suffering crop losses owing to incessant untimely rains causing inundation and sudden wilt following the wet spell. In addition to untimely heavy rains, faulty over-irrigation practices can cause inundation in chilli crops. A flat landscape is common in most chilli growing areas of India, and it is difficult to drain excess water from fields. The primary cause of the chilli sudden wilt has been established by this group as the inundation period of more than 24 hours which creates hypoxic conditions in rhizospere leading to the rapid loss of turgidity in the leaves and collapse of the whole plant in 2-4 days [2]. Though the primary cause is stagnant water, yet the soil-borne fungus Fusarium oxysporum further accelerates plant mortality.
Under waterlogged conditions, soils diffuse oxygen at a rate 104 times slower than air, and the process of respiration by roots of plants and soil microbes rapidly exhausts oxygen levels, causing plants to undergo various morphological, metabolic, physiological and transcriptional changes [3]. Consequently, there is significant reduction in root and shoot dry matter, root volume, leaf area, transpiration and leads to poor yield. In response to inundated environment, the plants adopt various mechanisms for tolerating hypoxic and anoxic conditions which include formation of adventitious roots near the surface and brace roots, formation of aerenchyma cells (internal gas spaces) in root cortex that helps in gas exchange in flooding conditions. The defence gene products include various defensive enzymes like Polyphenol Oxidase (PPO) and Peroxidase (POX) which catalyze the lignin formation and Phenylalanine Ammonia Lyase (PAL) which is involved in biosynthesis of phenolics, phytoalexins and reinforcement of cell wall constituents. Plants respond to flooding with adjustments in growth, biomass accumulation and allocation, and with anatomical and morphological modifications in roots, stems and leaves. When a highly virulent pathogen or severe abiotic stress attack the host plant, these defense mechanisms fail, suppressing or overriding the plant's resistance reaction [4].
Sudden wilt of chillies is a complex condition usually triggered by amalgamation of biotic and abiotic stresses has not been studied systematically [5]. The present study aimed to identify biochemical and physiological changes in sudden wilt affected chilli plants to gain a deeper understanding of this disorder.
Induction of sudden wilt in chilli and collection of plant organs
The studies were carried out in the experimental field area of the department of plant pathology, Punjab agricultural university, Ludhiana, India (latitude: 30.8987411 and longitude: 75.7955394) during the year 2020-2021. Sudden wilt susceptible cultivar CH-27 was raised in earthen pots of 24 cm diameter (1 plant/pot) containing soil autoclaved at 15 psi for 60 minutes for three consecutive days [6]. Artificial inoculations of chilli plants were carried out at stage 703 (Fruit size 0.5 cm–1 cm, elongation of pedicel). For inoculation, Fusarium oxysporum isolated from infected chilli plants was mass multiplied on pre-soaked chickpea seeds in 500 ml erlenmeyer flasks (125 gm/flask). With the help of a lead pencil, about 15 cm deep hole was made in the soil around the plant base along four geographical directions (North, South, East and West) and 4 g of mass multiplied inoculum was placed in each hole (16 g per pot). Potted plants were kept under normal irrigation conditions for seven days before imposing abiotic stress. For inducing abiotic stress, chilli plants were subjected to inundation for 48 hours by repeated irrigations to keep at least 6 cm of standing water above soil surface at 75 days after transplanting. Healthy check was maintained in pots filled with uninoculated sterilized soil without inundation stress with normal irrigation schedule (≈400 ml water/pot/day) [7]. As soon as the symptoms of sudden wilt syndrome were evident, the three test organs of chilli plant viz. roots, leaves and fruits, were collected from diseased and healthy plants for further assays.
Assessment of biochemical parameters associated with sudden wilt of chilli
Estimation of total phenolic content: For the estimation of total phenols, 0.3 g the three test tissues were homogenized with 5 ml of methanol for 60 minutes followed by filtration of refluxed material. Then, the total volume was made to 5 ml with the help of methanol. In the test tubes, 0.5 ml of methanolic extract was taken and evaporated till dryness, to which 6.5 ml of distilled water was added. Folin-phenol reagent (0.5 ml) was added and mixed well, followed by the addition of 1 ml of sodium carbonate after 5 minutes [8]. The contents were incubated at room temperature for 1 hour and the blue colour so formed was measured at 760 nm. The estimation of the phenolic compounds was carried out in three replications. Total phenolic content was calculated from the standard curve obtained by using different concentrations of gallic acid (10 μg-50 μg).
Estimation of total flavonoid content: For the assessment of total flavonoids, 0.5 ml of extract was evaporated until dryness followed by the addition of 0.1 M methanolic aluminum chloride (1.25 ml). The yellow colour obtained was measured at 420 nm and the flavonoids content was determined by using the standard curve prepared by using different concentrations of rutin (40 μg-200 μg) (Figure 1).
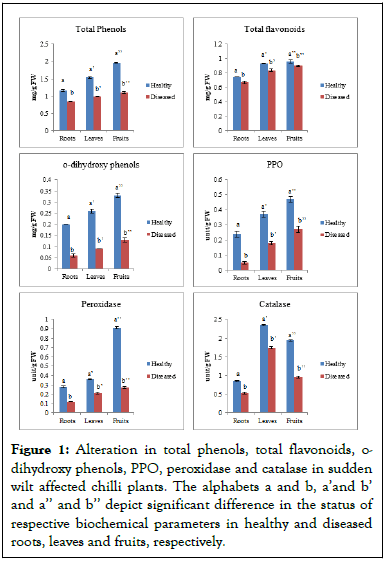
Figure 1: Alteration in total phenols, total flavonoids, o-dihydroxy phenols, PPO, peroxidase and catalase in sudden wilt affected chilli plants. The alphabets a and b, a’and b’ and a’’ and b’’ depict significant difference in the status of respective biochemical parameters in healthy and diseased roots, leaves and fruits, respectively.
Estimation of ortho-dihydroxy phenols content: To measure the o-dihydroxy phenols, 3 ml of methanolic extract was evaporated until dryness followed by addition of 1 ml distilled water, 0.3 ml of 10% TCA, 1 ml of 10% sodium tungstate, 0.5 ml of 0.5 N HCl and 1 ml of 0.5% freshly prepared sodium nitrate [9]. The yellow colour obtained was measured at 540 nm and the o-dihydroxy phenol content was determined by using the standard curve prepared by using different concentrations of catechol (5 μg-40 μg).
Estimation of ascorbic acid content: To assess the content of ascorbic acid, the tissue (0.1 g) was crushed in 1.3 ml of metaphosphoric acid followed by centrifugation at 10,000 xg for 10 minutes. The debris was discarded and the supernatant was used for the assay of ascorbic acid. Further, 200 μl of supernatant, 200 μl EDTA, 400 μl FeCl, freshly prepared in buffer, 400 μl TCA, 0.4 ml o-phosphoric acid and 0.4 ml bipyridyl were added followed by incubation at 40°C for half an hour [10]. The light pink colour obtained was read at 525 nm and ascorbate content was calculated from the ascorbic acid (5 μg-25 ug) standard.
Estimation of peroxidase activity: For the estimation of peroxidase activity, the tissue (0.1 g) was homogenised with 1.5 ml of 100 mm sodium phosphate buffer (pH 7.5) containing 1 mm EDTA, 1% PVP, 10 mM B-mercaptoethanol followed by centrifugation at 10,000 xg for 15 min at 4°C and clear supernatant was used to measure enzyme activity. The reaction mixture contained 3 ml of 50 mM guaiacol, 0.05 ml of enzyme extract and 0.1 ml of 0.8 M H2O2. The reaction was initiated by adding 1 ml of H2O2 and the change in optical density was measured at 470 nm after every 30 seconds till 3 minutes. Peroxidase activity was presented as an increase in absorbance/min/mg protein.
Estimation of catalase activity: For the estimation of catalase activity, the tissue (0.1 g) was homogenized with 2 ml of 50 mM sodium phosphate buffer (pH 7.5) containing 1% polyvinyl pyrrolidone followed by centrifugation at 10,000 xg at 4°C for 20 min and the clear supernatant was collected to determine enzyme activity. The reaction mixture was composed of 1.8 ml of 50 mm sodium phosphate buffer (pH 7.5) and 0.2 ml enzyme extract in the spectrophotometric cuvette [11]. The reaction was started by adding 1 ml of H2O2. The absorbance was measured at 240 nm after every 30 seconds upto 3 minutes. The activity was expressed as nmoles of H2O2 decomposed/min/mg protein.
Estimation of polyphenol oxidase activity: For the estimation of polyphenol oxidase activity, plant sample of 0.2 g was extracted with 1.5 ml of 100 mm sodium phosphate buffer (pH 6.8). Further, the plant tissue extract was centrifuged at 10,000 xg at 4°C for 25 minutes and the supernatant was used for enzyme assay. In the cuvette, 1 ml of 1000 mm sodium phosphate buffer (pH 6.8), 0.5 ml of 4 methyl catechol (0.1 M) were added along with 0.5 ml of enzyme extract [12]. The optical density was read at 410 nm after an interval of 30 seconds up to 3 minutes. The PPO activity was expressed as units/min/mg of protein.
Estimation of phenylalanine ammonia lyase activity: For the estimating Phenylalanine Ammonia Lyase (PAL) activity, the required tissue (0.1 g) was crushed with 2 ml of chilled 100 mm Tris HCI buffer (pH 7.5) containing 5 mm B-mercaptoethanol followed by centrifugation at 10,000 g at 4°C for half an hour and supernatant was collected for estimation of PAL. The reaction mixture consisting of 2.5 ml of 30 mm phenylalanine prepared in 50 mm sodium borate buffer (pH 8.8) and 0.1 ml of enzyme extract was incubated at 37°C for an hour [13]. The reaction was then stopped by adding the 0.3 ml of 5 N HCl. In control, the reaction was terminated immediately without giving any incubation period. The cinnamic acid formed was assayed at 290 nm against water blank. The activity of PAL was measured from the standard curve which was prepared by using a varying range of cinnamic acid (5 μg-40 μg). The activity was represented as ug of cinnamic acid formed/min/mg protein.
Estimation of tyrosine ammonia lyase activity: For Tyrosine Ammonia Lyase (TAL) activity, the required tissue (0.1 g) was crushed with 2 ml of chilled 100 mm Tris HCI buffer (pH 7.5) containing 5 mM B-mercaptoethanol followed by centrifugation at 10,000 xg at 4°C for half an hour and supernatant was collected for estimation of TAL. The reaction mixture comprising of 1 ml of 33 μM tyrosine, 1.35 ml of 50 mm of sodium borate buffer (pH 8.8) and 50 μl of enzyme extract was incubated at 37°C for an hour. The reaction was then stopped by adding 100 ul of 5 N HCl. In control, the reaction was terminated immediately without giving any incubation period. The enzyme activity was estimated at 310 nm.
Estimation of hydrogen peroxide: To measure the content of Hydrogen Peroxide (H2O2), the tissue (0.2 g) was crushed in 3 ml of chilled 0.1 % TCA. The mixture was then centrifuged at 10,000 g for 25 minutes and the supernatant was used for the determination of H2O2 content. In a cuvette, 0.5 ml of supernatant, 0.5 ml of 50 mm potassium phosphate buffer (pH 7.0) and 1 ml of potassium iodide were added and the intensity of pale color developed was read at 390 nm against the reagent blank which contained 1 ml of 50 mm potassium phosphate buffer (pH 7.0) and 1 ml of KI. The H2O, content was calculated from the standard curve prepared by using variable concentrations of H2O2 (25 μ-100 μ moles).
Estimation of malondialdehyde: To measure the Malondialdehyde (MDA) content, the tissue (0.2 g) was crushed in 3 ml of 5% TCA followed by centrifugation at 10,000 xg for 25 minutes at ambient temperature. Then the supernatant was collected for estimation of MDA content. An equal amounts of supernatant and 20% TCA containing 0.5% TBA were mixed and the mixture was heated at 95°C in a water bath for half an hour and then cooled on ice followed by centrifugation at 10,000 xg for 10 minutes. The optical density of yellow color was measured at 532 nm and 600 nm. The extinction coefficient for MDA (155/mm/cm) was used to calculate the MDA content.
Estimation of lignin: To quantify the lignin content, the tissue (0.1 g) was crushed in 95% ethanol followed by centrifugation at 10,000 g for 20 minutes at ambient temperature. Then the supernatant was discarded and pellet was washed 3 times with 95% ethanol. This pellet was given 2 times washing with ethanol:hexane (1:2). Further, the pellet was dried at 45°C overnight. On next day, the dried pellet was washed with solution of 25% acetyl bromide in acetic acid followed by incubation at 70°C for 30 mins and cooled at room temperature. Take 100 μl of extract and then add 180 μl NaOH followed by 20 μl of Hydroxylamine HCl and mix 1.6 ml of acetic acid. Further, centrifuge it for 15 min and read at 280 nm.
Estimation of proline: For gauging the proline content, the leaf tissue (100 mg) was crushed in 4 ml of 3% aqueous sulphosalicylic acid and filtered with whatman filter paper. Then the filtrate was collected for estimation of proline content. In the test tubes, 3 ml extract, 2 ml ninhydrin reagent and 2 ml acetic acid was kept in boiling water bath at a temperature of 100°C for 1 hr). The reaction was stopped by placing on ice and further, 4 ml toluene was added in all the test tubes. The pink colour obtained by taking the upper layer from the test tube was read at 520 nm (Figure 2).
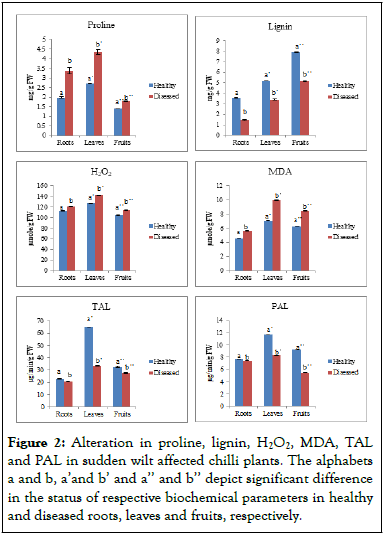
Figure 2: Alteration in proline, lignin, H2O2, MDA, TAL and PAL in sudden wilt affected chilli plants. The alphabets a and b, a’and b’ and a’’ and b’’ depict significant difference in the status of respective biochemical parameters in healthy and diseased roots, leaves and fruits, respectively.
Estimation of total sugars: To measure the content of total soluble sugars, the tissue sample from the plant tissue was washed with distilled water. 100 mg of fresh tissue was weighed and put in a 100 ml conical flask. The extraction of sugars was carried out in 80% ethanol [14]. It was further followed by double extraction in 70 % ethanol over boiling water bath every time for 20 min. It was then subjected to centrifugation at a speed of 5000 rpm for at least 5 min and extracts obtained were pooled. The final volume of pooled extracts was made upto 10 ml by concentrating extracts. It was followed by the addition of 0.2 ml of lead acetate and sodium oxalate. The purpose of adding sodium oxalate is to remove extra lead ions. The supernatant from this extract obtained after centrifugation was used to estimate total soluble sugars. 0.9 ml of distilled water was added to the test tube containing 0.1 ml of sugar extract. After this, 1 ml of 5% phenol solution was added to the test tube making the volume 2 ml. This was followed by the addition of 5 ml chilled H2SO4 to the tube containing solutions of extract and phenol after 5 min. The tube was cooled under tap water for 20 mins. On cooling, an orange-brown coloration was developed. It was read at 490 nm against a blank containing all reagents except the sample extract on spectrophotometer. The preparation of blank was done by adding 1 ml of 5% phenol to 5 ml of chilled H2SO4 and 1 ml of distilled water. Glucose was used as a standard (10 μg/ml-100 μg/ml) against which the concentration of total soluble sugars in tissue sample was calculated.
Estimation of total proteins: For the estimation of total proteins, the grounding of sample tissue from the plant tissue was done in 0.5 ml of 0.1 N NaOH. This was followed by centrifugation at a speed of 5000 rpm for 10 min. The extraction procedure was carried out twice with the supernatant obtained and the final volume was made to 10 ml. 1 ml of Trichloroacetic Acid (TCA) (15%) was added to the aliquot and kept for one day at 4°C. The above mixture was subjected to centrifugation at a speed of 5000 rpm for 20 mins. Precipitates were dissolved in 0.1 N NaOH. Further, 5 ml reagent C was added to 1 ml of carefully diluted sample extract solution. Vortex was done to mix the contents thoroughly. Then the mixture was left still at room temperature for 10 min. This was followed by the addition of 0.5 ml of reagent D [15]. The contents were mixed thoroughly. A blue coloration was developed in the mixture that was read at 520 nm on a spectrophotometer after keeping the mixture undisturbed for 30 min at room temperature against a blank. The blank contained all the above contents except the sample extract. Various concentrations of Bovine Serum Albumin (BSA) were used to prepare the standard curve (Figure 3).
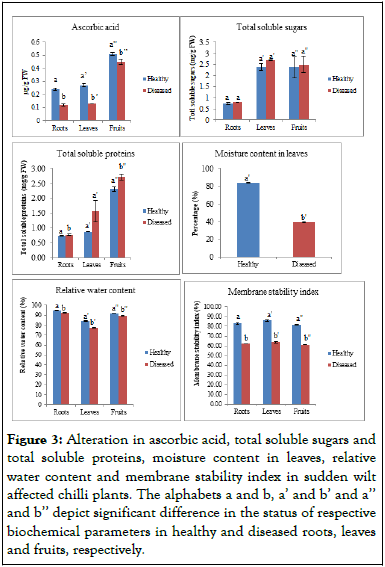
Figure 3: Alteration in ascorbic acid, total soluble sugars and total soluble proteins, moisture content in leaves, relative water content and membrane stability index in sudden wilt affected chilli plants. The alphabets a and b, a’ and b’ and a’’ and b’’ depict significant difference in the status of respective biochemical parameters in healthy and diseased roots, leaves and fruits, respectively.
Estimation of moisture content in leaves: For estimating the moisture content in leaves, the leaf samples from healthy and diseased plants were weighed and air-dried from all replications.
These were then subjected to oven-drying at 60°C for 24 hrs. These samples were weighed again. The formula used for calculating moisture content in leaves is as follows:

Estimation of relative water content: Relative water content was measured from 100 mg of leaves using the protocol given by Weatherly. Following detachment from the plant, tissue was immersed in a pre-weighed test tube containing distilled water. The increase in weight of the test tubes was used to calculate the Fresh Weight (FW) of tissue by reweighing the tubes. The tissue saturated with water was weighed again after 28 h in order to obtain its Turgid Weight (TW). It was then subjected to oven-drying for 48 h at 70°C. The formula used for calculating RWC is as follows:
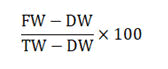
Where;
FW=Stands for the fresh weight of tissue.
TW=Stands for the turgid weight of tissue.
DW=Stands for the dry weight of tissue.
Estimation of chlorophyll A and B: Total chlorophyll content was estimated using the procedure given by Anderson and Boardman. 0.1 g portion of freshly harvested and finely chopped leaf tissues were immersed in a test tube containing 10 ml of Dimethyl Sulphoxide (DMSO, (CH3)2SO). These were then shifted to the oven at 60°C for approximately two hours for pigment extraction. After that, the extract was allowed to cool at room temperature for some time and then the absorbance was read at 645 nm and 665 nm on a spectrophotometer. The chlorophyll content was determined using the following formula:
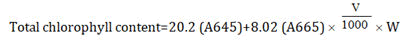
Where,
A645=Absorbance at 645 nm.
A665=Absorbance at 665 nm.
V=Total volume of extract (ml).
W=Weight of the sample (gm).
Estimation of membrane stability index: The membrane stability index was recorded for leaves, fruit and roots of chilli plant as per the standard procedure. Tissue was made free of endogenous and exogenous electrolytes by washing with deionized water. The amount of tissue taken for analysis was 100 mg from florets and leaves of each replication of all treatments. This tissue was incubated in 25 ml distilled water at 25°C for 30 minutes [16]. The conductivity was measured on the conductivity meter to estimate the electrolyte leakage into the distilled water which was used as an incubation medium. After that, the tissue sample was boiled for 30 min and its conductivity was measured again. The Membrane Stability Index (MSI) was calculated with the following formula:
MSI=(1-C1/C2) × 100
Estimation of gas exchange parameters: For the estimation of gas exchange parameters Nnet Photosynthesis (Pn), transpiration rate (E) and stomatal conductance (gs) were determined from healthy and diseased leaves at the fruiting stage (90 DAP-100 DAP) by using Infra-Red Gas Analyzer (IRGA, model LCA-4). An IRGA takes advantage of the absorbance of CO2 molecules at a wavelength of 4260 nm caused by the stretching vibrations of the C=O double bonds. Portable IRGAs have been engineered to take relatively rapid readings from air that has passed over a leaf surface. Thus the rate of gas exchange can be measured on an intact leaf under field conditions [17]. A sophisticated IRGA can measure (and even control) the concentration of CO2 and humidity entering the leaf chamber, the intensity of the incident light and the CO2 concentration exiting the chamber. At the same time, it can calculate and record photosynthesis rates using an on-board computer. With such an IRGA, one can determine photosynthetic light response curves for a plant species under specific temperatures and CO2 concentrations (BSCI 1510L literature and stats guide: Infrared gas analysis) (Figure 4) [18].
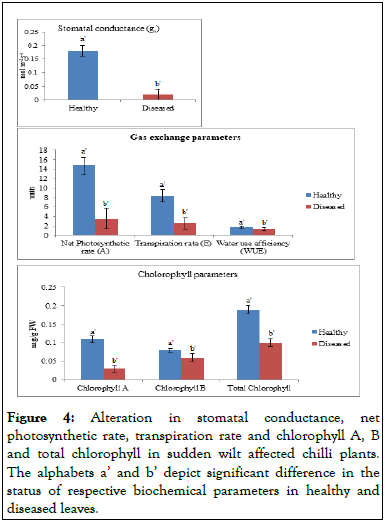
Figure 4: Alteration in stomatal conductance, net photosynthetic rate, transpiration rate and chlorophyll A, B and total chlorophyll in sudden wilt affected chilli plants. The alphabets a’ and b’ depict significant difference in the status of respective biochemical parameters in healthy and diseased leaves.
Source: Units-Net photosynthetic rate (A) = μmol m-2s-1,Transpiration rate (E) = mol m-2s-1
Data analysis
Independent t-test was employed to analyze biochemical and physiological parameters associated with sudden wilt of chilli using SPSS 16.0 software (p ≤ 0.05). The data presented in bar diagrams depict the mean ± standard deviation of triplicate samples.
Status of total phenols, o-dihydroxy phenols, flavonoids and ascorbic acid
For healthy plants, the total phenol content was 1.15 mg/g, 1.54 mg/g and 1.97 mg/g FW in roots, leaves and fruits respectively, while for diseased plants, the total phenol levels were 0.85 mg/g, 0.97 mg/g and 1.11 mg/g FW, respectively. The results indicated a significant decline in phenol content in all the three test organs of diseased plants as compared to healthy plants. The content of o-dihydroxy phenols in roots, leaves and fruits of healthy and diseased plants was 0.19 mg/g, 0.26 mg/g and 0.33 mg/g FW and 0.06 mg/g, 0.09 mg/g and 0.12 mg/g FW, respectively. Phenolic compounds were lower in sudden wilt affected plants of susceptible cultivar CH-27, which could not cope-up with the amalgamated stress imposed by inundation and Fusarium oxysporum. Phenolic compounds are derived from the phenylpropanoid pathway, which confers resistance to plants against a variety of biotic and abiotic stresses as well as scavenge Reactive Oxygen Species (ROS). Biochemical analysis of the test organs revealed significantly higher content of flavonoids in healthy chilli plants in contrast to diseased plants. The total flavonoid content in roots, leaves and fruits of healthy and diseased plants was 0.73 mg/g, 0.93 mg/g and 0.96 mg/g FW and 0.67 mg/g, 0.84 mg/g and 0.89 mg/g FW, respectively [19]. Flavonoids have a high antioxidant potential to combat reactive radicals, their reduced levels in response to sudden wilt in CH-27 plants demonstrate the inability of cells to combat the stress.
Chilli peppers are rich in ascorbic acid, a compound that is important as an antioxidant and determines its nutritive value. The results in this study showed a significant difference between the ascorbic acid content of healthy and diseased plants. The ascorbic acid content in roots, leaves and fruits of healthy plant was 0.24 mg/g, 0.27 mg/g and 0.51 μg/g respectively, whereas, in case of diseased plants, the content of ascorbic acid was 0.12 mg/g, 0.13 mg/g and 0.45 μg/g in roots, leaves and fruits respectively.
Profile of peroxidase, polyphenol oxidase, catalase, hydrogen peroxide and malondialdehyde as affected by sudden wilt of chilli
The activity of POX in roots, leaves and fruits of healthy plants was 0.28 unit/g, 0.36 unit/g and 0.90 unit/g FW, respectively. But, in case of diseased plants, the activity declined to 0.12 unit/g, 0.20 unit/g and 0.27 unit/g FW, respectively. The healthy and diseased plants showed the variable response with respect to Catalase (CAT) activity also. The activity of CAT in roots, leaves and fruits of healthy plants was adjudged to be 0.86 unit/g, 2.35 unit/g and 1.94 unit/g FW, respectively, In case of diseased plants, the activity of catalase reduced to 0.53 unit/g, 1.74 unit/g and 0.95 unit/g FW in roots, leaves and fruits, respectively. Thus, observations indicate that sudden wilt syndrome significantly stifles POX and CAT activity, impairing the plants' ability to cope with the stress. In addition to catalyzing lignin biosynthesis, Peroxidase (POX) participates in the oxidation of phenols, the conversion of hydroxy cinnamaldehyde to free radicals and binding of monomers and polysaccharides during suberization. The enzyme Catalase (CAT) converts H2O2 to H2O, preventing the accumulation of H2O2.
Plants make Hydrogen Peroxide (H2O2) as an important non radical reactive oxygen species in their normal metabolism. However, when excessive amounts of H2O2 accumulate as a result of biotic or abiotic stresses, plants can suffer oxidative stress or cell death. There was significant difference in the content of H2O2 in healthy and sudden wilt affected plants. The content of H2O2 was lower in healthy plants in comparison to diseased plants. In healthy plants, the H2O2 content in roots, leaves and fruits was 112.90 μmole/g, 127.21 μmole/g and 104.89 μmole/g FW, respectively. In contrast, the H2O2 content of diseased plants was higher in roots, leaves, and fruits, with values of 112.90 mol/g, 127.2 mol/g, and 104.89 mol/g FW, respectively. Malondialdehyde (MDA) level is most commonly used as an indicator of oxidative stress and also shows the amount of lipid peroxidation triggered by stressed conditions in plants. In diseased plants, MDA content was significantly higher than that in healthy plants, as indicated by the statistical analysis. The MDA content in roots, leaves and fruits of healthy plants was 4.59 μmole/g, 7.10 μmole/g and 6.30 μmole/g FW, respectively, and the diseased plants exhibited an increase in MDA content viz., 5.64 μmole/g, 9.99 μmole/g and 8.46 μmole/g FW in roots, leaves and fruits, respectively.
Profile of enzymes of phenol metabolism
Healthy and sudden wilt affected plants displayed a significant difference in PAL activity. In healthy plants, the activity of PAL in roots, leaves and fruits was estimated to be 7.72 μg/min/g, 11.7 μg/min/g and 9.24 μg/min/g FW, respectively. In contrast, in diseased plants, the level of the enzyme was recorded to be on lower side with values of 7.42 μg/min/g, 8.32 μg/min/g and 5.50 μg/min/g FW in roots, leaves and fruits, respectively. Similarly, there was a significant difference in the Tyrosine Ammonia Lyase (TAL) activity of healthy and diseased plants. The activity of TAL in roots, leaves and fruits of healthy plants was 22.88 μg/min/g, 65.10 μg/min/g and 32.49 μg/min/g FW, respectively. In diseased plants, the activity of TAL was 20.56 μg/min/g, 33.60 μg/min/g and 27.59 μg/min/g FW in roots, leaves and fruits, respectively. Plants synthesize key metabolites necessary for growth and reproduction through the phenylpropanoid pathway. The enzyme of phenol metabolism includes Phenylalanine Ammonia Lyase (PAL), Tyrosine Ammonia Lyase (TAL) and Polyphenol Oxidase (PPO). While PAL catalyses the conversion of l-phenylalanine to transcinnamic acid and ammonia, TAL plays an important role in catalyzing the tyrosine deamination to p-coumaric acid which converts to caffeic acid, which is a phenolic precursor.
The activity of PPO showed a significant difference in healthy and diseased plants. In healthy plants of CH-27, the activity of PPO in roots, leaves and fruits was 0.24 unit/g, 0.37 unit/g and 0.47 unit/g FW, respectively. On the other hand, the activity of PPO in diseased plants declined to 0.05 unit/g, 0.18 unit/g and 0.26 unit/g FW, respectively. Polyphenol Oxidase (PPO) has the property to oxidize the phenolic compounds to quinones, which are more toxic to pathogens in comparison to original phenolic compounds. The quinones are highly reactive and can polymerize with nucleophilic chains of amino acids. Further, these quinones crosslink with proteins causing the reduction in availability of such proteins, leading to the appearance of brown pigments in the damaged or affected plant tissues (Figure 5).
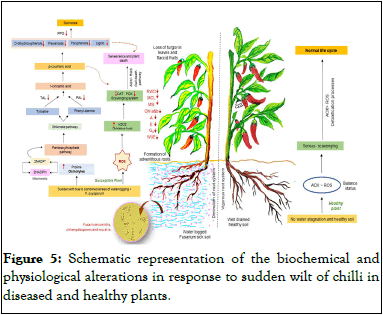
Figure 5: Schematic representation of the biochemical and physiological alterations in response to sudden wilt of chilli in diseased and healthy plants.
Lignin and proline
Lignin, an important secondary metabolite that is synthesized by phenylalanine/tyrosine metabolic pathway, enhances the cell wall rigidity of plant, hydrophobicity and mineral transport through the vascular bundles of plants. It acts as an important barrier against the entry of pathogens. In the present study, there was a significant difference between the lignin content of healthy and diseased plants. The content of lignin in roots, leaves and fruits of healthy plants was 3.57 mg/g, 5.15 mg/g and 7.93 mg/g, respectively. On the other hand, there was decrease in diseased plants with lignin content of 1.46 mg/g, 3.39 mg/g and 5.13 mg/g in roots, leaves and fruits, respectively.
There was a significant difference in the proline content of healthy and diseased plants of chilli. The proline content in roots, leaves and fruits of healthy plants was 1.97 mg/g, 2.70 mg/g and 1.39 mg/g FW, respectively while, in the case of diseased plants it increased to 3.38 mg/g, 4.34 mg/g and 1.79 mg/g FW in roots, leaves and fruits, respectively. The amino acid proline, a plant molecule with several functions, plays an important role in stressed plants as an osmolyte, chelator of metals, signaling molecule, and antioxidative defense component.
Total sugars and total proteins
In the present study, the total sugar content didn’t show significant difference in healthy and diseased plants. The content of TSS was lower in healthy plants in comparison to the diseased plants. The total sugar content in roots, leaves and fruits of healthy plants was 0.74 mg/g, 2.39 mg/g and 2.37 mg/g, respectively. But, in the diseased plant, the content was 0.79 mg/g, 2.69 mg/g and 2.50 mg/g FW in roots, leaves and fruits, respectively. The total sugar content is the main photosynthate and essential form of temporary storage and carbon metabolism. It helps in carbon metabolism and has a close relationship with the process of photosynthesis. It acts as a sign of supply ability in leaves and it also reflects ability and transformation of grains for using assimilates. Proteins are the important consituents of plants and are the polymer of amino acid, having high molecular weight. The data of Total Soluble Protein (TSP) didn’t showed significant difference in the healthy and diseased plants. The soluble proteins were lower in healthy plants in comparison to the healthy plants. The total soluble protein in roots, leaves and fruits of healthy plants was 0.73 mg/g, 0.87 mg/g and 2.31 mg/g FW, respectively. But, in case of diseased plants, the total soluble protein was 0.77 mg/g mg/g, 1.56 and 2.71 mg/g FW, respectively.
Relative water content, moisture content in leaves and membrane stability index
Relative Water Content (RWC) is a measure of plants' water status. It is an important metric for determining plant's capacity to tolerate dehydration. In the current study, there was a pronounced difference between healthy and diseased plants in terms of water content. Diseased plants had a lower RWC than healthy plants. RWC in roots, leaves, and fruits in healthy plants was 94.61%, 84.11%, and 91.82% respectively. However, in diseased plants, the values of RWC dropped to 92.26%, 77.10%, and 89.71% in roots, leaves, and fruits, respectively. Reduced water uptake and loss in turgidity of leaves might explain the reduction of RWC registered in inundated plants. There was a significant difference between the relative water content of healthy and diseased plants. The moisture content in leaves was also recorded and there was significant difference between the moisture content of healthy and diseased plants. The content of moisture was higher in the healthy plants in comparison to the diseased plants. The moisture content in leaves of healthy and diseased plants was 84.15% and 39.39%, respectively. Membrane Stability Index (MSI) is a measure to estimate the permeability and leakage of ions from the cell membrane of the plant cell. It is also used as a screen test for stress tolerance. In the present study, the data of membrane stability index showed a significant difference in healthy and diseased plants. The MSI of healthy plants was higher in comparison to the diseased plants. The MSI in roots, leaves and fruits of healthy plants was 82.74%, 85.80% and 81.60%, respectively, but, in case of diseased plants, there was a decrease in MSI with the values of 62.06%, 63.80% and 61.04% in roots, leaves and fruits, respectively.
Chlorophyll A, B and gas exchange parameters
Both the chlorophyll A and B showed a significant difference between the healthy and diseased plants. The chlorophyll content was higher in the leaves of healthy plants in comparison to the diseased plants. The chlorophyll A and B content in leaves of healthy plants was 0.11 and 0.08 respectively. But, the content of chlorophyll A and B in leaves of diseased plants was 0.03 and 0.06 respectively. The chlorophyll A and B are the major types of chlorophyll found in plants. These are involved in the process of photosynthesis but differ in their role. The principal pigment in the process of photosynthesis is chlorophyll A, while chlorophyll B acts as accessory pigment which collects the energy for passing it to the primary pigment (chlorophyll A).
The various gas exchange parameters were evaluated which includes net photosynthetic rate (A), transpiration rate (E), Water Use Efficiency (WUE) and stomatal conductance (gs). All these parameters showed significant difference between the healthy and diseased plants. The net photosynthetic rate (A) was higher in healthy plants (14.93) in comparison to diseased plants (8.34). The transpiration rate (E) in leaves of healthy and diseased plants was 8.34 and 2.49, respectively. Also, the Water Use Efficiency (WUE) was estimated by calculating the ratio of net photosynthetic rate and transpiration rate. The WUE in leaves of healthy and diseased plants was 1.79 and 1.36, respectively. Since, the process of photosynthesis is dependent on the chlorophyll content. So, with the decrease in chlorophyll content, there was reduction in the net photosynthetic rate in diseased plants. Also, the decrease in relative water content resulted in the reduction of transpiration rate. Stomatal conductance (gs) is the amount of gas exchange (CO2 uptake) and transpiration (loss of water) through the stomata which is determined by the degree of stomatal aperture (the physical resistance for the gas movement between the leaf interior and the air). Hence, it depicts the density, size and rate of stomatal opening. By having more open stomata, a greater amount of conductance is possible, implying a faster rate of transpiration and photosynthesis. In the present study, the stomatal conductance of healthy and diseased leaf showed a significant difference with higher stomatal conductance in healthy leaves (0.18) in comparison to the diseased leaves (0.02).
The present study attempts to gain insight into the biochemical and physiological aspects of sudden wilt syndrome, a relatively new and economically important problem affecting chillies. The primary cause of the disorder has been established by this group as inundation of 24 hours-48 hours which creates hypoxic conditions in rhizosphere leading to the rapid loss of turgidity in the leaves and collapse of the whole plant [20]. In affected plants, mortality is further enhanced by Fusarium oxysporum, a fungus readily present in wilt-sick soils. Plants alter their metabolism to develop specialized strategies to cope with different stresses by triggering a variety of biochemical and physiological changes associated with stress signaling and activating their defenses. Such responses occurring in chilli plant in response to an amalgamation of abiotic and biotic stress has been summarized in Figure. In contrast to the healthy plants, the sudden wilt affected chilli plants had lower levels of total phenols, o-dihydroxyphenols, flavonoids, ascorbic acid, peroxidase, polyphenol oxidase, catalase, phenylalanine ammonia lyase, tyrosine ammonia lyase, polyphenol oxidase, lignin, relative water content, moisture stability index, moisture content in leaves, chlorophyll A and B and the gas exchange parameters (net photosynthesis, transpiration rate and stomatal conductance), all evidence of an impaired defense system. In contrast, hydrogen peroxide and malondialdehyde were elevated, two markers of oxidative stress, along with proline, suggesting the emergence of adaptive mechanisms to maintain osmoregulation within the diseased plants.
In addition, in stressed chilli plants, there was loss in Moisture Content of Leaves (MCL), Relative Water Content (RWC), Membrane Stability Index (MSI) and chlorophyll content (chl A and B). As a result of loss in chlorophyll content, the net photosynthetic rate (A) was also hampered which caused the reduction of transpiration rate (E) and stomatal conductance (gs). Also, there was an enhancement in ROS activity that led to the oxidative burst as evidenced by the elevated levels of H2O2. Thus, the scavenging system in susceptible plants was negatively affected, resulting in a reduction in catalase and peroxidase activities. When the level of antioxidants is much lower than ROS in diseased plants, the cell death pathway might have been triggered, resulting in senescence and plant death. In parallel, there is an elevation of proline osmolytes, which may have disrupted the pentose phosphate and shikimate pathways as demonstrated by the significant decrease in PAL and TAL activities. The level of cinnamic acid and p-coumaric acid may have decreased due to a reduction in the activity of PAL and TAL, respectively. Since t-cinnamic acid and p-coumaric acid are responsible for the production of phenolic compounds (polyphenols, o-dihydroxyphenols, flavonoids, lignin) and an important defense related phenol metabolism enzyme (PPO), senescence and plant mortality are caused by a significant decrease in levels of such compounds. PPO and phenol compounds decrease quinone formation, an essential defense compound. In addition, the reduction in p-coumaric acid causes senescence and plant death to occur more quickly. In well-drained and healthy soils where disease-free plants’ root system is vigorously growing without any water stagnation, there is a balance of anti-oxidants and ROS. Excessive ROS are nullified through the detoxification pathway by anti-oxidants in the sensor-scavenging mechanism.
To our knowledge, this is the first systematic attempt to study the underlying biochemical and physiological changes that lead to sudden chilli wilting.
Sudden wilt syndrome is an unexplored and economically important disorder primarily caused by inundation and aggravated by Fusarium oxysporum infection. This study examines physiological and biochemical changes in three test organs of chilli namely, roots, leaves, and fruits, when exposed to sudden wilt syndrome. The sudden wilt-affected plants had lower levels of total phenols, o-dihydroxyphenols, flavonoids, ascorbic acid, peroxidase, polyphenol oxidase, catalase, phenylalanine ammonia lyase, tyrosine ammonia lyase, polyphenol oxidase, lignin, moisture content in leaves, relative water content, membrane stability Index, chlorophyll A and B and the gas exchange parameters (net photosynthesis, transpiration rate and stomatal conductance), all indicating towards an impaired defense system. However, hydrogen peroxide and malondialdehyde levels as well as proline levels increased, indicating adaptive mechanisms that maintain normal osmoregulation. Healthy plants had similar levels of total sugars and proteins to diseased plants. These findings can help to provide the foundation for designing mitigating strategy of sudden wilt syndrome in field.
P.S. induction of stress and disease creation in pots, conducted the biochemical and physiological assays in lab, data compilation and wrote the manuscript.
S.J. conceived the idea and designed the experiment, supervised the work with lab assays, experimental design, data evaluation and critically revised the manuscript.
R.D.B. and R.R. Assisted in data evaluation and laboratory assays.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors are grateful to the Punjab agricultural university, Ludhiana for providing the facilities to conduct the above experiments.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Jain S, Salaria P, Rani R, Bhardwaj RD (2023) Physio-Biochemical Responses of Chilli Peppers (Capsicum annuum L.) to Sudden Wilt Syndrome. J Plant Biochem Physiol. 11:280.
Received: 25-Jul-2023, Manuscript No. JPBP-22-20760; Editor assigned: 27-Jul-2023, Pre QC No. JPBP-22-20760 (PQ); Reviewed: 10-Aug-2023, QC No. JPBP-22-20760; Revised: 17-Aug-2023, Manuscript No. JPBP-22-20760 (R); Published: 24-Aug-2023 , DOI: 10.35841/2329-9029.23.11.280
Copyright: © 2023 Jain S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.