International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Mini Review - (2021)Volume 9, Issue 2
Schwann cells (SCs) are specialized glial cells that wrap and protect axons in the peripheral nervous system (PNS). There are a variety of SCs including non-myelinating SCs such as Remak SCs, perisynaptic/ terminal SCs, repair/ Bünger SCs and nociceptive SCs that surround axons or axon terminals without forming myelin sheath. Non-myelinating SCs play important roles in proper myelin development and maintenance, repair and regeneration after injury, and nociception. A recently published work showed that transient receptor potential vanilloid 4 (TRPV4), a Ca2+-permeable permeable cation channel, is exclusively expressed in non-myelinating SCs rather than myelinating SCs and plays an important role in nerve demyelination in response to injury. In this short communication, we are going to review the recent studies and discuss the possible significance of TRPV4 channels in the non-myelinating SCs.
Transient Receptor Potential Vanilloid 4 (TRPV4); Schwann Cells (SCs); Non-myelinating Schwann cells (Non-myelinating SCs); Demyelination; Myelination; Nerve injury; Pain sensation
Schwann cells (SCs) are primary peripheral glial cells and cover the surface of axons in peripheral nervous system. SCs are divided into myelinating SCs and non-myelinating SCs (Remak SCs, perisynaptic/terminal SCs, repair/ Bünger SCs and nociceptive SCs). All SCs derive from multipotent progenitor cells of the neural crest. The fate decision mechanism of SCs to become myelinating cells or to form non-myelinating SCs is not fully understood, although the plasticity of SCs in various studies is recognized [1]. The non-myelinating SCs differentiation is governed, at least in part, by neuronal cues, especially by the signaling pathway neuregulin 1 type III (Nrg1-III)/ErbB2/ErbB3 receptor cascades. However, a number of cell-autonomous genes also contribute to SCs differentiation toward non-myelinating SCs such as gamma-aminobutyric acid type B1 receptor (GABBR1) [2]. Myelinating SCs ensheathe large-diameter axons (Aα, Aβ or Aδ fiber) with multiple layers to form myelin sheath while nonmyelinating SCs surround multiple small-caliber axons or nerve terminals by a single layer of the plasma membrane and form remark bundles. The latter non-myelinating SCs provide crucial trophic supports for developing neurons and profoundly influence axonal properties during development. For instance, Remak SCs surrounding small-diameter axons provide trophic supports to the surrounding axons while perisynaptic/terminal SCs overlaying the neuromuscular junctions are involved in synaptic transmission and nerve regeneration. Repair/Bünger SCs respond to nerve injury by converting themselves into an immature-like phenotype to promote axon regeneration and repair. Nociceptive SCs are newly discovered non-myelinating SCs that attach nociceptive nerve endings to form a glio-neural complex in skin [3]. Although non-myelinating SCs don’t generate myelin sheath, these cells are critical for the proper development and function of peripheral nervous system. Feng et al. for the first time reported that TRP vanilloid channel subfamily 4 (TRPV4) is functionally expressed in non-myelinating SCs and that TRPV4 expression is increased in SCs following demyelination in response to sciatic nerve injury, which enhances nerve remyelination and functional recovery (Figure 1) [4].
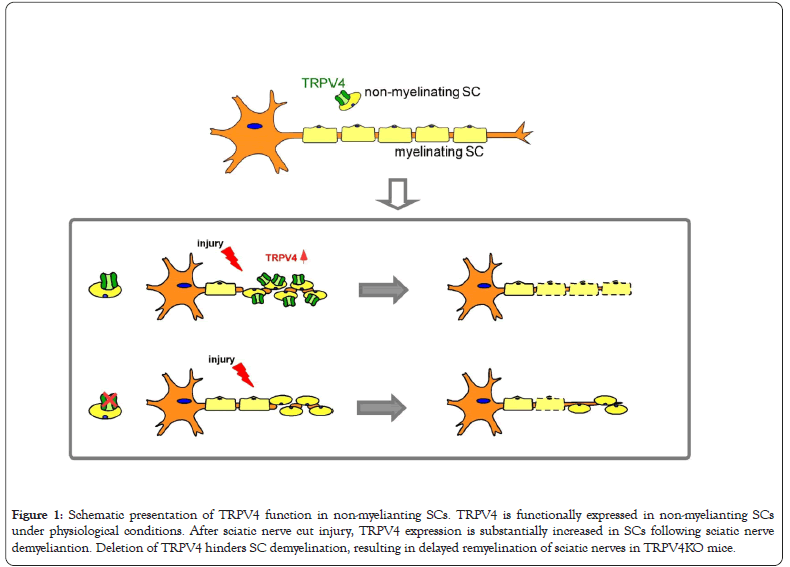
Figure 1:Schematic presentation of TRPV4 function in non-myelianting SCs. TRPV4 is functionally expressed in non-myelianting SCs under physiological conditions. After sciatic nerve cut injury, TRPV4 expression is substantially increased in SCs following sciatic nerve demyeliantion. Deletion of TRPV4 hinders SC demyelination, resulting in delayed remyelination of sciatic nerves in TRPV4KO mice.
TRPV4 is a member of TRPV channel subfamily which is activated by various stimuli, including mechanical stimulation, moderate thermal, osmolarity, some endogenous chemicals such as anandamide, arachidonic acid and its epoxyeicosatrienoic acid metabolites, as well as by a number of exogenous chemical ligands or UVB [5,6]. This channel is widely expressed and activated in various neurons and glial cells in the nervous system. In microglia, TRPV4 is involved in LPS-induced activation [7]. TRPV4 was also shown to be expressed and function in the proliferation of oligodendrocyte precursor cells [8]. Satellite glial cells, which are derived from the neural crest of the embryo during development like SCs, have also been reported to functionally express TRPV4 where it may regulate inflammatory pain by enhancing the purinergic signaling pathway [9]. Feng et al. showed that TRPV4-deficient (TRPV4KO) mice can generate normal myelin sheath during peripheral nerve development in the physiological condition.
Given that non-myelinating SCs are essential for nerve repair process in response to peripheral nerve injury, Feng et al. investigated the function of TRPV4 in the mouse model of Wallerian degeneration. The distal nerve degenerates following sciatic nerve injury. SCs respond to loss of axons by breaking down myelin sheath, followed by downregulation of myelinrelated genes. At the same time, autophagic processes are activated in these demyelinating SCs [named as repair or Bünger SCs). Demyelinating SCs cooperate with recruited macrophages to clear axon and myelin sheath fragments which potentially inhibit axonal growth [10]. On the other hand, demyelinating SCs secrete several neurotrophic factors to promote neuronal survival and axon growth. Then, these SCs form tracks that guide regenerating axons back to their targets. Once SC-axon contact is re-established, repair SCs differentiate and remyelinate the regenerated axons, which is necessary for functional recovery of peripheral nerves. SCs remain highly plastic, and respond adaptively to injury and trigger repair process to maintain axolemmal organization and neuronal health during these processes [11]. Feng et al. found that nerve demyelination is blocked in response to injury in TRPV4KO mice, resulting in the delay of nerve remyelination, which indicates that TRPV4 can enhance nerve demyelination after injury in non-myelinating SCs.
It is interesting that TRPV4 is not involved in normal myelin development, but it is crucial for nerve repair in response to nerve injury. It is possible that TRPV4 has a function in SC autophagy that mediates myelin breakdown and myelin debris clearance following sciatic nerve cut-induced Wallerian degeneration, while these autophagic processes are absent during myelin development. Previous reports have suggested that TRPV4 induces autophagy in several cell types. For instance, TRPV4 induces autophagy through the AKT signaling pathway, potentially by regulating Ca2+ levels and osmotic pressure in hepatic stellate cells [12], and TRPV4 was also reported to increase autophagy-related proteins during osteoclast differentiation by activating the Ca2+-calcineurin-NFATc1 pathway [13]. It is well known that the control of myelin breakdown and myelin debris clearance is a central step in nerve injury and pathology [14]. Myelin debris clearance must rapidly occur over the course of 7-14 days after injury [14-16], and robust and efficient removal of myelin debris is an important contributor to nerve regeneration and remyelination. TRPV4 deficiency likely impairs the myelin breakdown and debris clearance function by SCs, resulting in residual myelin debris in the distal stumps after injury, and this could interfere with remyelination and functional recovery. Another possibility is that TRPV4 could participate in pathways that maintain the appropriate levels of myelin structural proteins in response to injury. For example, c-Jun is a negative regulator of myelination during development while it not only suppresses the expression of myelin genes, but also promotes myelinophagy and upregulates some important neurotrophic factors and cell surface proteins to support neuronal survival and axon growth during repair [17]. It will be interesting to determine the molecular pathways how TRPV4 in SCs is involved in the process of sciatic nerve cut-induced Wallerian degeneration in the future.
In addition to the critical role of non-myelinating SCs in proper myelin development and nerve repair after injury, nonmyelinating SCs also function in pain transmission. Dysfunctions of non-myelinating SCs were reported to cause neuropathic pain. For example, a specific deletion of LDL receptor-related protein-1 [LRP1] in SCs results in impaired Remak SC ensheathment and mild hypomyelination, which causes mechanical allodynia in mice even in the absence of injury [18]. Moreover, deletion of GABBR1 in SCs causes increased numbers of c-fibers in Remak bundles, leading to a hyperalgesic and allodynic phenotypes in mice [2]. Although TRPV4 was reported to mediate nociceptive responses to hypotonic stimuli [19], the function of TRPV4 in non-myelinating SCs in neuropathic pain remains unknown. It’s well known that axon demyeliantion induces chronic neuropathic pain after peripheral nerve injury. The result that loss of TRPV4 hindered axon demyelination found by Feng et al. suggests that TRPV4 in SCs is involved in peripheral nerve injury-induced neuropathic pain. Interestingly, a recent report discovered a type of non-myelinating SCs, called nociceptive SCs, that directly interact with nociceptive nerve terminals innervating the epidermis where nociceptive signals are initiated. And these nociceptive SCs especially contribute to the detection of mechanical stimuli [3]. However, it is not known which kinds of ion channel of SCs detect mechanical stimuli and transduce the nociceptive stimuli into electrical signals [3,20]. The report that attenuation of nociceptive TRPV1 channel promotes the peripheral regeneration after injury [21,22] could raise the potential interaction of two TRPV channels, TRPV1 and TRPV4 for nerve regeneration, although TRPV1 is not functionally expressed in SCs [4]. It is intriguing to determine whether TRPV4 is involved in the detection of mechanical stimuli in SCs. Further investigations of TRPV4 together with other TRP channels in SCs and neurons would be needed to understand the molecular pathways in which TRPV4 is involved regarding SC plasticity and nociception.
This work was supported by MEXT KAKENHI Grant-in-Aid for Scientific Research Grant and Scientific Research on Innovative Areas ‘Thermal Biology’ to M.T.
The authors declare no competing interests.
Citation: Feng X, Song X, Tominaga M (2021) Physiological Significance of TRPV4 Channels in Non-Myelinating Schwann Cells. Int J Phys Med Rehabil. 9:588.
Received: 27-Jan-2021 Accepted: 10-Feb-2021 Published: 17-Mar-2021 , DOI: 10.35248/2329-9096.21.9.588
Copyright: © 2021 Feng X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.