Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2024)Volume 15, Issue 1
Despite the rising interest in avocado leaves and seeds health benefits, little or no research has been conducted on both their phytochemical profiles in conjunction with molecular docking investigations, particularly in relation to its antioxidant activity. Utilizing phytochemical screening molecular docking and Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) research. This study investigates the antioxidant properties of Avocado Leaves (AVL) and Avocado Seeds (AVS). Results show that AVS has a high presence of flavonoids (+++), terpenoids (+++), but a low presence of phenols (+), while AVL has a high presence of tannins (+++) and phenols (++). Molecular docking studies validate two AVL (L01 and L02) and two AVS (S02 and S03) compounds based on binding affinity and interactions with 2 Retrograde Urethrogram (RGU).pdb, 3 Multinodular Goiter (MNG).pdb and 2 Vessel Wall Imaging (VWI).pdb protein targets. ADMET studies indicate that AVL and AVS extracts have favorable bioavailability and health safety characteristics.
ADMET; Avocado; Molecular docking; Phytochemical screening
Avocado (Persea americana mill. cultivar hass) is becoming more and more popular in the field of nutrition and health. This well-liked fruit has sparked a great deal of interest due to its intriguing green attractiveness. The benefits of avocados go far beyond their delicious flavor, since their leaves and seeds are a veritable source of phytochemical compounds. Avocado (Persea americana mill. cultivar hass) have been widely discussed on their phytochemical composition and activities in a variety of health disorders, including anti-inflammatory, anti-hypertensive and anti-diabetic activities. Avocado flourish in subtropical and tropical climes, preferring moderate temperatures and well-drained soils [1-7] (Figure 1).
Figure 1: (A): Avocado tree; (B): Avocado leaves; (C): Avocado seed.
Recent studies have focused on the association between anti-inflammatory, anti-hypertensive and anti-diabetic compounds found in AVL and AVS. Several studies revealed that the bioactive chemicals contained in AVL and AVS may have anti-inflammatory effects that might aid in the reduction of inflammation, which is a critical role in chronic illnesses such as diabetes, hypertension and other metabolic disorders [8]. However further research is needed to corroborate these findings and support the potential use of AVL and AVS extracts as functional foods or nutraceuticals with health-promoting qualities.
Theoretically Puspita, et al., [9] and Kusuma, et al., [10] had both used molecular docking modeling to explore the binding affinity and interaction of AVL and AVS molecules with various protein targets linked with anti-inflammatory, anti-hypertensive and anti-diabetic properties. Furthermore, Bhatt, et al., [11] in an avocado research applied ADMET property prediction to evaluate the possible bioavailability, distribution, metabolism, excretion and safety profiles of AVL and AVS extracts, giving critical information for their prospective usage as functional foods or nutraceuticals. Despite increased interest in the health benefits of avocado leaves and seeds little study has been undertaken on their phytochemical profiles and molecular docking studies, particularly in connection to their antioxidant activity implications.
The goal of this study is to look at the phytochemical profiles of avocado leaves and seeds to perform molecular docking simulations so as to determine whether they have any anti-inflammatory, anti-hypertensive or anti-diabetic characteristics. The study also intends to predict the ADMET features of avocado leaf and seed extracts. The main objective is to get insights into the antioxidant capacity and health benefits of avocado leaves and seeds by combining phytochemical profiling, molecular docking and ADMET predictions. The findings of this study might help researchers better understand the bioactive chemicals found in avocado leaves and seeds, as well as their potential uses in functional foods and nutraceuticals.
Phytochemical screening
Extraction of avocado leaves and seed: The hass avocado specie, Persea americana mill. cultivar hass, was the specie of avocado selected for this study. Persea americana mill. cultivar hass is one of the most widely grown and consumed avocado varieties globally, known for its creamy texture, rich flavor and dark purple to black pebbly skin when ripe.
The leaves and ripening avocados (Persea americana mill. cultivar hass), harvested in the dry season (March-April) were purchased from the NIHORT farm in Ibadan, Nigeria. The avocado fruit was peeled and the soft fruit's seeds were removed. Following thorough washing, the seeds were broken, the leaves were washed in clean water and both were dried in a food dehydrator (Excalibur, model-4926T) for 48 hours at 50°C. The dried seeds and leaves were then pulverized with a grinder (Rico Mixer Grinder (RGM) "1701") and sieved to make a fine powder. The extraction methodology was tested using a modified method reported by Chaalal, et al., [12]. 2 grams of avocado seed and leaf powder were combined with 40 milliliters (mL) of 80% methanol solvent. Following 3 mins of stirring, the mixture was incubated for 30 minutes in a water bath at 50°C. Before filtering, the extracts were centrifuged at 4000 rpm for 15 minutes.
Qualitative analysis
The active phytochemicals tannins, saponins, flavonoids, terpenoids, phenols and sterols were identified by qualitative analysis of avocado seed and leaf methanol extract.
Assessment of tannins
Ferric chloride test: To 0.5 g of each sample, 10 ml of 5% ferric chloride was added. The presence of tannins was indicated by the appearance of greenish black or dark blue color.
Assessment of saponins
Each sample (5 mg) was mixed with 5 ml of distilled water in the volumetric flask. After this accumulation, the sample was mixed vigorously for almost 15 min. The presence of saponins in the test sample was revealed by the creation of a soapy layer.
Assessment of flavonoids
Each sample (2 mg) was added in 2 ml of 2 N NaOH. The presence of yellow color was the confirmation sign for flavonoids.
Assessment of terpenoids
Each sample (1 mg) was taken in the test tube and 5 ml of each CHCl3 and concentrated H2SO4 was added to the samples. Formation of brown layer in the midst of other two layers indicated the presence of terpenoids.
Assessment of phenols
Each sample (0.5 mg) was taken in test tube, 1 ml of distilled water and 5% FeCl3 was added in it. The formation of blue color confirms the presence of phenols.
Assessment of sterols
Each sample extracts (1 ml) was taken in test tube, 3 ml of CHCl3 was added and then 0.5 ml concentrated sulphuric acid was carefully dispensed along the walls of the test tube (Salkowski test). The presence of sterols was revealed by the emergence of reddish color in the lower layer.
Total phenolic and total flavonoid contents
Total phenolic was assessed and determined by a method described by Chandra, et al., [13]. The test sample (0.5 ml) was mixed with 1 ml of distilled water and 2.5 ml of Folin-Ciocalteu’s reagent (5:1) in a volumetric flask. After 5 min, 2 ml of saturated sodium carbonate solution (8% w/v) in water was added to the mixture and the volume was made up to 10 ml with distilled water. The reaction was held in the dark for 30 minutes before centrifuging and the absorbance of the blue color was measured from separate samples at 765 nm. The phenolic content was measured in milligrams of Gallic Acid Equivalent (GAE) per gram (mg GAE/g) of extracts. Total flavonoid was performed by a method described by Batool, et al., [2]. The reaction mixture was prepared in a test tube by adding 0.5 ml sample, 0.15 ml NaNO2 (0.5 mol/l), 0.3 M AlCl3.6H2O and 5 ml methanol (30%). After 5 minutes, 2 ml of 1 M NaOH was added to the mixture, which was then read at 506 nm wavelength. Whereas, the flavonoid content was measured in milligrams of Catechin Equivalent (CE) per gram (mg CE/g) of extracts.
Fluorescence Recovery After Photobleaching (FRAP)
A 2.5 ml of 0.2 M phosphate buffer and 2.5 ml of 1% potassium ferricyanide was added to 1 ml of extract in a test tube. Reaction mixture was incubated at 50°C for 20 minutes, 2.5 ml of 10% of trichloroacetic acid was added before centrifuging at 2000 rpm for 10 minutes. 2.5 ml of distilled water was added to the supernatant. After adding 0.5 ml of 0.1% Fecl3, reaction mixture was incubated in dark at room temperature for 10 minutes before reading absorbance at 700 nm.

2,2-Azino-Bis-(3-Ethylbenzothiazoline-6-Sulfonic acid) (ABTS)
ABTS radical scavenging activity was conducted using method as adopted with slight modification. ABTS radical cation was produced by reacting 7 mM ABTS with 2.45 mM potassium persulphate then stored in dark for 12-16 hrs at room temperature. To 0.1 ml of extract, 3 ml of ABTS radical added. Absorbance was read at 734 nm using UV-VIS spectrophotometer (model T80) after incubating in dark for 30 minutes.
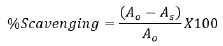
Where Ao=Absorbance of control and As=Absorbance of sample.
2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
DPPH free radical scavenging was done according to method by Kumar, et al., [14] 3 ml of 2,2-diphenyl-1-picryhydrazyl was added to 1 ml of sample extract then stored in dark for 30 minutes before reading absorbance was read using Ultraviolet(UV)-Visible(VIS) spectrophotometer (model T80) at 517 nm.
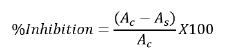
Where Ac=Absorbance of control and As=Absorbance of sample.
Gas Chromatography (GC)-Mass Spectroscopy (MS) analysis
The Agilent 6890 N gas chromatography, which has an auto sampler and is coupled to a mass spectrometer, was used for this study. A one microliter of the sample was injected in the pulsed splitless mode on a fused silica column with a film thickness of 0.15 micrometers and a 30 m x 0.25 mm Internal Diameter (ID). The column head pressure was kept at 20 psi while helium gas was employed as the carrier gas, resulting in a constant flow rate of 1 ml/min. The column temperature was first maintained at 55°C for 0.4 minutes, then increased to 200°C at a rate of 25°C per minute, then to 280°C at a rate of 8°C per minute and finally to 300°C at a rate of 25°C per minute, kept for 2 minutes.
Ligand preparation
In this study, ligands were created by modeling their chemical structures in ChemDraw® Ultra version 12.0.2.1076 software [15]. The Molecular Mechanics Force Field (MM-FF 99) technique, which was developed using the Avogadro version 1.2.0 software program, was used to optimize the ligands. We were able to compute the energy of the ligands and adjust their structures to increase their stability and interactions with target proteins using this technique [16,17].
Receptor preparation
In this inquiry, receptors were retrieved and downloaded from the rcsb.org portal for further processing. The receptors were cleaned with the BIOVIA® DS version 2021 software program, which removed crystallized water and the heteroatoms associated with it, resulting in a clean receptor structure. After that, the cleaned receptor structure was stored in .pdb format. The receptor structure was then optimized, polar hydrogen added and Geister charge assigned using the Chimera® version 35.15.1443 software program. The improved receptor structure was then stored in the autodock' .pdb qt format, where it was ready for docking. This process resulted in a clean, optimized receptor structure that was acceptable for molecular docking simulations [18-20].
Receptor used for this study are
• Human dipeptydylpeptase (IV) (2RGU.pdb) [21].
• Human peroxiredoxin (V) (3MNG.pdb) [22].
• Human serine/threonine kinase (2VWI.pdb) [23].
Molecular docking studies
The Autodock Vina tool, version 1.2.0 was used via Pyrx software to perform molecular docking simulations in this investigation [24,25]. The docking methodology consisted of verifying the protein's active site by docking known ligands retrieved from the Research Collaboratory for Structural Bioinformatics (RCSB) portal. This guaranteed that the selected compounds were assessed at the receptor's active site. Based on the validation results, a box size was established for each receptor and documented in Table 1. The screening procedure was then initiated, with the determined box size for each receptor. For each simulation cycle, the exhaustiveness or run time was set to 10. The docked score was recorded and compared to the standard drug after each simulation round. This technique enabled an accurate assessment of the compounds' binding affinity as well as the identification of prospective lead compounds for further development (Table 1).
| Receptor | Box size dimension |
|---|---|
| 2RGU | Center: 43.7 × 54.4 × 34.64 |
| Dimension: 39.8 × 32.46 × 29.45 | |
| 3MNG | Center: 9.093 × 43.73 × 19.68 |
| Dimension: 37.34 × 32.46 × 36.83 | |
| 2VWI | Center: 93.87 × 157.39 × -21.04 |
| Dimension: 41.25 × 40.79 × 42.76 |
Note: RGU: Retrograde Urethrogram; MNG: Multinodular Goiter; VWI: Vessel Wall Imaging.
Table 1: Box-dimension used for the molecular docking simulation.
Visualization and plotting tools
All docking visulization were done on the BIOVIA® DS version 2021. Graphical plots were done using two python libraries called pandas, Matplotlib and Scipy on LibreOffice version 7.5 [26,27].
ADMET property studies
ADMET lab version 2.0 was used to conduct pharmacokinetics, toxicity and drug-likeness studies to predict the ADMET properties of compounds obtained from both leave and seed compounds [28]. The server calculated Ames’ oral toxicity, water solubility, brain-blood barrier crossing, cytochrome' inhibition, the lipinski rule of five (Lipinski, 2002 and other properties. The five conditions for lipinski violation includes: Molecular weight ≤ 500, mlogP ≤ 4.15, number of N and O ≤ 10, NH ≤ 5 and OH ≤ 5 [29].
GC-MS analysis
Supplementary data, 3-4 and 1-2 show the gas chromatogram of AVL and AVS compounds extracts, respectively. Results show how AVL detected more abundant compounds than AVS. It’s observed that from retention time 4 mins-14 mins and 20 mins-21 mins, AVL showed great abundance of similar compounds to AVS.
Phytochemical screening
Qualitative phytochemical screening: The results of qualitative phytochemical screening for AVL and AVS revealed distinct phytochemical profiles. AVS was found to be high in flavonoids (+++), terpenoids (+++) and low in phenols (+), whereas AVL was high in tannins (+++) and low in phenols (+). Saponins were absent in AVL (+) but present in minor quantities in AVS. Steroids were not found in either the AVL or the AVS.
Quantitative phytochemical screening: This study conducted a quantitative phytochemical screening of avocado seeds to determine variations in the phenolic and flavonoid contents of AVL and AVS. The phenolic and flavonoid content were measured in milligrams of Gallic Acid Equivalent (GAE) per gram (mg GAE/g) and milligrams of Catechin Equivalent(CE) per gram (mg CE/g), respectively and the results are shown in Table 2.
| Phenolic content (mg/ GAE g) | Flavonoid content (mg/ QE g) | |
|---|---|---|
| AVL | 48.083 ± 1.176a,c | 0.71 ± 0.07 |
| AVS | 16.11 ± 0.78c | 3.58 ± 0.38 |
Note: AVL: Avacado Leaves; AVS: Avacado Seeds; GAE: Gallic Acid Equivalent; QE: Quercetin Equivalent; (a-c):Trails.
Table 2: Quantitative mean and standard deviation data of AVL and AVS phenolic, flavonoid, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) and EC50 activity.
As shown in Table 2, AVL has a significantly higher phenolic content (48.083 ± 1.176 mg GAE/g) than AVS (16.11 ± 0.71 mg GAE/g), while AVS has a significantly higher flavonoid content (3.58 ± 0.38 mg CE/g) than AVL (0.71 ± 0.78 mg CE/g) (Table 2).
Table 3, however, shows the antioxidant assays (DPPH, ABTS and FRAP). Results show that DPPH radical inhibition increases in the order of AVL (46.36%) to AVS (70.97%), which is comparable to that of ascorbic acid at 93.28%. The Effective Concentration (EC) 50 for the DPPH antioxidant activity also reflects a higher antioxidant activity for AVS (0.35) than AVL (0.54). Furthermore, AVS inhibited ABTS radicals by 47.35%, better than 6.67% and comparable to vitamin C’ (57.13%). Likewise, the EC50 activity shows higher activity in AVS (0.14) than in AVL (0.96). On the other hand, AVL inhibited FRAP radicals by 11.07% more than AVS (3.66%), while vitamin C inhibited FRAP radicals by 81.55%. As expected, the EC50 activity shows a similar trend as the FRAP inhibition pattern of AVL, AVS and vitamin C (Table 3).
| DPPH (% inhibition) | EC50 (DPPH) (mg/mL) | ABTS (% inhibition) | EC50 (ABTS) (mg/mL) | FRAP (% inhibition) | EC50 (FRAP) (mg/mL) | |
|---|---|---|---|---|---|---|
| Avocado leaves AVL | 46.36a,b | 0.54 | 6.67a,b | 0.96a,b | 11.07a,b | 3.07c |
| Avocado seeds AVS | 70.97a,b,c | 0.35 | 47.35a,b | 0.14 | 3.66a,c | 9.25a,b,c |
| Vitamin C | 93.28a,b | 0.27 | 57.13a,c | 0.11 | 81.55b,c | 0.42 |
Note: DPPH: 2,2-Diphenyl-1-Picrylhydrazyl; EC: Effective Concentration; ABTS: 2,2′-Azinobis-(3-Ethylbenzothiazoline)-6-Sulfonic Acid; FRAP: Fluorescence Recovery After Photobleaching; (a-c) : Trails.
Table 3: Antioxidant (DPPH, ABTS and FRAP) activity data of AVL and AVS.
Molecular docking studies: A computational approach known as molecular docking predicts the binding orientation and affinity of a small molecule ligand to a target protein. The purpose of this study was to gain insight into the interaction between avocado leaf and seed components and a therapeutic target protein, as well as to forecast the potential effectiveness and specificity of avocado leaf and seed as a drug candidate [30,31]. Validation visualization was performed prior to docking to confirm the active sites of each receptor. The validation compounds were not docked of this study but were only used to visualize the docked complex downloaded from the RCSB web portal. The validation compounds were discovered at the protein receptor's active site, while their binding sites are circled in red. The dock-score for the validation compounds ranges from -9.2 (kcal/mol) to -8.2 (kcal/mol).
The compound-residue interaction for the validation compound in Figure 2 helps to understand that all other docking score calculations for all receptor-AVL and receptor-AVS compounds were performed precisely at the active site range. The docked score for the compounds from the avocado leaves ranges from -8.5 to -4.3 kcal/mol for 2RGU receptor, -7.7 to -3.7 kcal/mol for 2VWI receptor and -6.5 to -3.7 kcal/mol for 3MNG. The docked score for compounds obtained from the seed ranges from -8.1 to -5.1 kcal/mol for 2RGU, -7.9 to -4.8 kcal/mol for 2VWI and -4.1 to -6.8 kcal/mol for 3MNG receptor, respectively.
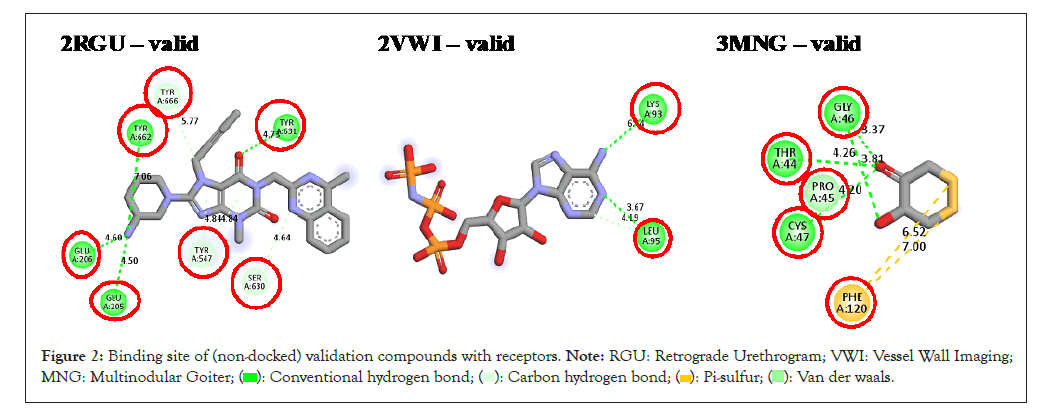
Figure 2: Binding site of (non-docked) validation compounds with receptors. Note: RGU: Retrograde Urethrogram; VWI: Vessel Wall Imaging;
MNG: Multinodular Goiter; 
For the anti-diabetes studies, the docking scores for leave compounds L01, L02, L03, L04 and seed compounds S01, S02, S03 and S04 all outperformed metformin (-4.9 kcal/mol) (2RGU). Using the human serine/threonine kinase (2VWI) receptor, L01, L02 and seed compounds, S02 and S03 are avocado compounds that outperformed amlodipine's binding score (-6.6 kcal/mol). The avocado compounds that outperformed diclofenac's binding score (-5.5 kcal/mol) in anti-inflammatory studies using the human PrxV (3MNG) receptor were leave compounds L01 and L02 and seed compounds S02 and S03. The docking score results show that avocado leaf compounds L01 and L02, along with avocado seed compounds S02 and S03, are the validated compounds by the docking process (Figure 2 and Table 4).
| Compounds | Names | Chemical structures | Binding affinity (kcal/mol) | ||
|---|---|---|---|---|---|
| 2RGU | 2VWI | 3MNG | |||
| L01 | Stigmasta-5,22-dien-3-ol | 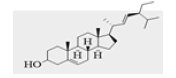 |
-8.5 | -7.7 | -6.5 |
| L02 | campestenol | 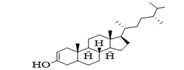 |
-7.9 | -7.4 | -6.3 |
| L03 | 2(1H)-naphthalenone | 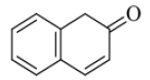 |
-6 | -6.2 | -5.3 |
| L04 | n-hexadecanoic acid |  |
-5.6 | -5 | -4.3 |
| L05 | 2-pyrrolidinone | 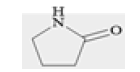 |
-4.3 | -3.7 | -3.7 |
| S01 | 9,17-octadecadienal | 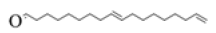 |
-5.1 | -4.8 | -4.1 |
| S02 | Ergost-5-en-3-ol | 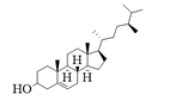 |
-8.1 | -7.9 | -6.8 |
| S03 | Stigmastan-3,5,22-trien | 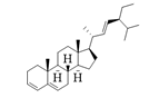 |
-8.1 | -7.5 | -7 |
| S04 | n-hexadecanoic acid |  |
-5.4 | -4.8 | -4.4 |
| Saturation Transfer Difference( STD) | Metformin, Diclofenac and Amlodipine | - | -4.9 | -6.6 | -5.5 |
| *VDATE | -8.9 | -8.2 | -9.2 | ||
Note: *VDATE: Validation compounds used to confirm the binding site of the protein structure; RGU: Retrograde Urethrogram; VWI: Vessel Wall Imaging; MNG: Multinodular Goiter.
Table 4: Docking scores of leave and seed compounds on human Dipeptidyl Peptidase-4 (DPP-4) (2rgu.pdb), human PrxV (3mng.pdb) and human serine/threonine kinase (2vwi.pdb) protein targets.
The compound-receptor interaction results for AVL and AVS, show that the length of the hydrogen, van der waals and other non-conventional hydrogen bonds formed for the avocado leave compounds did not exceed 6.8 Å length, which is in agreement with suggestions from Ahmed, et al., [32] and Falade, et al., [33] while it did not exceed 7.0 Å length for the seed compounds. This could be one of the reasons for the docking score's high binding energies (Figure 3 and Figure 4).
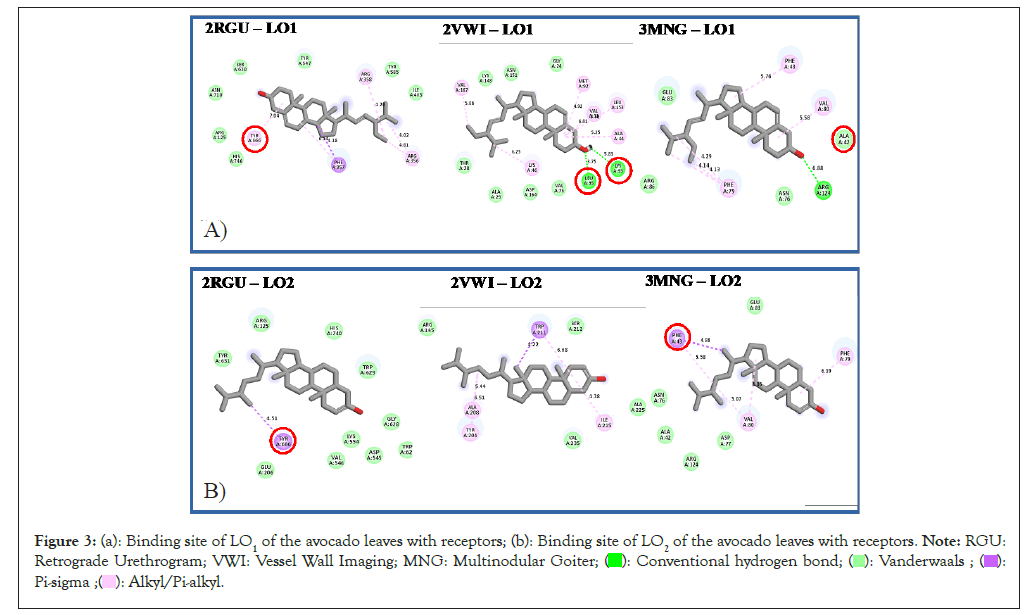
Figure 3: (a): Binding site of LO1 of the avocado leaves with receptors; (b): Binding site of LO2 of the avocado leaves with receptors. Note: RGU:
Retrograde Urethrogram; VWI: Vessel Wall Imaging; MNG: Multinodular Goiter; 
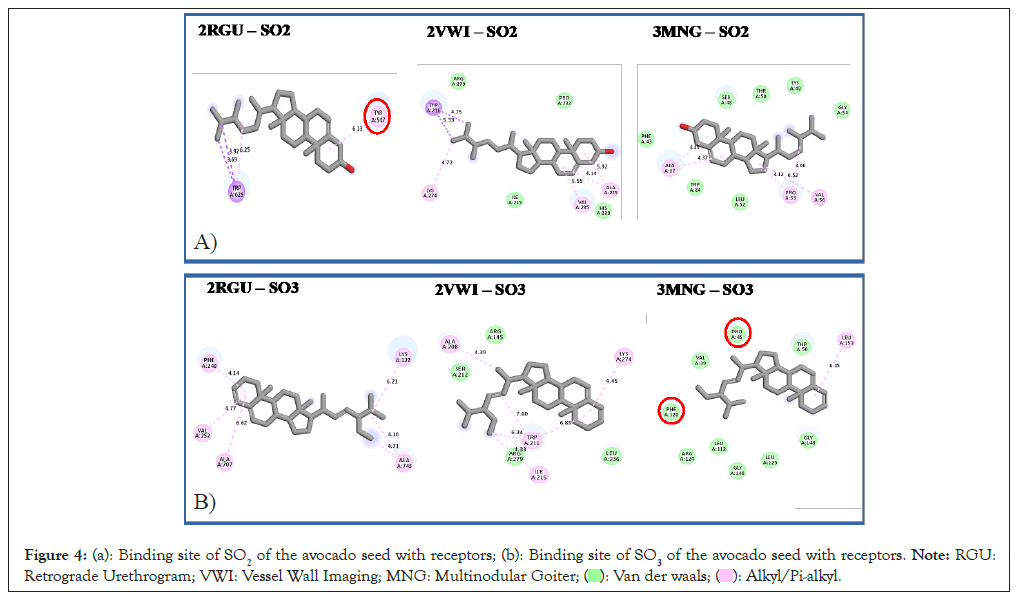
Figure 4: (a): Binding site of SO2 of the avocado seed with receptors; (b): Binding site of SO3 of the avocado seed with receptors. Note: RGU:
Retrograde Urethrogram; VWI: Vessel Wall Imaging; MNG: Multinodular Goiter; 
ADMET studies: Table 5 summarizes the findings of the Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) study. Previous studies, such as Falade, et al., [33], de Souza Neto, et al., [34] and van de Waterbeemd [35], have suggested a range of acceptable results for ADMET property studies. Using SMILE formula notations, Xiong, et al., [28] and Kiefer, et al., [36] successfully trained supervised models to predict the ADMET properties of compounds (Table 5).
| Compounds | Water solubility (mol/L) | HIA (%) | BBB | CYP inhibition? | Half-life (hours) | HERG blocker? | AMES toxicity | Lipinski |
|---|---|---|---|---|---|---|---|---|
| Violations? | ||||||||
| AVL | ||||||||
| L01 | -6.89 | >=90 | Negative | 44931 | 0.8 | Inactive | Negative | 0/5 |
| L02 | -6.77 | >=90 | Negative | 0/5 | 1.1 | Inactive | Negative | 0/5 |
| AVS | ||||||||
| S02 | -7.12 | >=90 | Negative | 0/5 | 1.2 | Inactive | Negative | 0/5 |
| S03 | -7.35 | >=90 | Negative | 44931 | 1.1 | Inactive | Negative | 0/5 |
| Standard drugs | ||||||||
| Metformin | -1.05 | >=90 | Negative | 0/5 | 2.2 | Inactive | Negative | 0/5 |
| Diclofenac | -4.77 | >=90 | Negative | 44931 | 2.8 | Inactive | Negative | 0/5 |
| Amlodipine | -2.71 | >=90 | Negative | 44931 | 2 | Inactive | Negative | 0/5 |
Note: HIA: Human Intestinal Absorption; BBB: Blood-Brain-Barrier; CYP: Cytochrome; HERG: Human Either-a-Go; AMES: Bruce Ames Toxicity test method; AVS: Avacado Seeds; AVL: Avacado Leaves.
Table 5: Absorption, Distribution, Metabolism and Excretion (ADME) property prediction for compounds identified in AVL and AVS with the current market drugs for hypertension (Amlodipine®), diabetes (Metformin®) and inflammation (Diclofenac®).
Solubility concentrations for L01 (-6.88), L02 (-6.765), S02 (-7.122) and S03 (-7.346) were found to be lower than the recommended range of -3.0 to -6.0 mol/L, as suggested by. Further dissolution techniques, such as decoction or alcohol extraction, can be used to optimize this abnormality for both the leaf and seed compounds. The human absorption rate was found to be good and safe for L01, L02, S02 and S03, indicating their potential as drug candidates. None of these four qualified compounds were found to cross the blood-brain barrier, indicating that they do not alter neurological functions or cause addiction in patients. According to Kiefer, et al., [36] Cytochrome P (CYP)450 inhibition for all drug candidates was found to be within the acceptable range of 1 violation. All potential drug candidates half-lives were found to be between 0.6 and 3 hours, as recommended by de Souza Neto, et al., [34]. All four drug candidates tested negative for hERG blockers, indicating that they do not have the potential to weaken the human heart, progressively. The AMES toxicity prediction test was negative for all drug candidates and the lipinski drug-like test confirmed that all four drug candidates were good enough as a drug candidate.
The presence of tannins in AVL indicates its potential therapeutic effects in the treatment of diseases such as cancer, diabetes and cardiovascular disease, as a result of its antioxidant and anti-inflammatory properties. Similarly, from Table 6, showing the phenols found in AVL and AVS are potent antioxidants that can protect cells from oxidative damage and reduce the risk of developing chronic diseases. AVS high flavonoid content suggests that it can protect cells from oxidative damage, reduce the risk of chronic diseases and provide anti-inflammatory, anticancer and neuroprotective effects [37,38]. Terpenoids are also found in high presence in AVS and in low concentrations in AVL, indicating that they are bioactive chemicals with anti-inflammatory, anticancer and antioxidant properties that have been linked to potential therapeutic benefits in the treatment of a variety of disorders such as asthma, arthritis and cancer. However, the absence of saponins in AVL suggests that it may be limited in its ability to provide anti-inflammatory, antioxidant and anticancer properties in comparison to AVS. It is worth noting, from Table 6 that neither AVL nor AVS contained any steroids. It can be infered from the qualitative phytochemical results that, that AVL and AVS may have different health benefits and therapeutic implications. However, further studies are needed to confirm these potential health benefits and to determine the best therapeutic doses (Table 6).
| Pytochemicals | Avocado leaves | Avocado seeds |
|---|---|---|
| Tannin | +++ | + |
| Saponin | - | + |
| Flavonoid | + | +++ |
| Terpenoid | + | +++ |
| Steroid | - | - |
| Phenol | ++ | + |
Note: (+++): Strongly present; (++): Mildly present; (+): Slightly present; (-): Absent.
Table 6: Qualitative analytical result for Avacado Leaves (AVL) and Avocado Seeds (AVS) compounds.
Phenols and flavonoids are phytochemical groups known for their antioxidant capabilities, quantitatively, the higher phenolic concentration in AVL suggests that it may have more antioxidant activity than AVS. The higher phenolic content of AVL may contribute to its potential therapeutic benefits in preventing chronic diseases caused by oxidative stress [39]. The higher flavonoid content of AVS, on the other hand suggests that it may have better antioxidant and health-promoting properties than AVL. This could be attributed to flavonoids' potent antioxidant and anti-inflammatory properties, which can protect against a variety of health problems. According to higher inhibition percentages and lower EC50 values in the DPPH and ABTS assays, AVS appeared to have more antioxidant activity than AVL, according to the results on Table 4. In spite of the fact that both considerably underperformed vitamin C in this assay, AVL showed stronger FRAP radical suppression than AVS. These disparities in antioxidant activity can be explained by understanding that different radical inhibition tests have different mechanisms of action. While the main objective of the DPPH and ABTS tests are to evaluate the capacity of antioxidants to scavenge particular stable radicals (DPPH and ABTS radicals), the FRAP assay evaluates the reducing capacity of antioxidants [40].
According to the study's findings, it can be inferred that AVL and AVS have distinct phenolic and flavonoid profiles, which may have therapeutic implications. Because of its higher phenolic concentration, AVL may be more effective as an anti-oxidant, whereas AVS may have better antioxidant and other health-promoting qualities due to its higher flavonoid concentration. However, more research is equally needed to confirm these potential health benefits and to determine the best therapeutic doses.
Ahmed, et al., [32] and Falade, et al., [33] established that a good docking score should not be less than the dock score of the measuring standard drug. Hence, it can be inferred from results from Table 5 that leave compounds L01 (Stigmasta-5,22-dien-3-ol), L02 (campestenol), L03 (2(1H)-Naphthalenone), L04 (n-Hexadecanoic acid) and seed compounds S01 (9,17-Octadecadienal), S02 (Ergost-5-en-3-ol), S03 (Stigmastan-3,5,22-trien) and S04 (n-Hexadecanoic acid) all has binding energies or scores that exceeds that of metformin (-4.9 kcal/mol) for the anti-diabetes studies (2RGU). For the anti-hypertension studies, using the human serine/threonine kinase (2VWI) receptor, L01 (Stigmasta-5,22-dien-3-ol), L02 (campestenol) and seed compounds, S02 (Ergost-5-en-3-ol) and S03 (Stigmastan-3,5,22-trien) are the avaocado compounds that exceeded the binding score of amlodipine’ (-6.6 kcal/mol). For the anti-inflammatory studies, using the human PrxV (3MNG) receptor, leave compounds, L01 (Stigmasta-5,22-dien-3-ol) and L02 (campestenol) and seed compounds, S02 (Ergost-5-en-3-ol) and S03 (Stigmastan-3,5,22-trien) are the avocado compounds that exceeded the binding score of diclofenac (-5.5 kcal/mol). Results from the docking score can be summarized that avocado leave compounds: L01 (Stigmasta-5,22-dien-3-ol) and L02 (campestenol) with avocado seed compounds, S02 (Ergost-5-en-3-ol) and S03 (Stigmastan-3,5,22-trien) are the validated compounds by the docking process. This can be an important reason for the high binding energies recorded in the docking score. This result is supported by Dzouemo, et al., [41] stated that the ligand-receptor interaction at the binding site of a potential drug candidate must not exceed 7.0 Å.
According to the findings of this study, Table 5 shows that all four drug candidates that passed the docking screening (L01, L02, S02 and S03) have the potential to be used in the treatment of diabetes, hypertension and inflammation in patients. According to the findings of this study, the therapeutical efficacy of avocado will range from diabetes to hypertension to inflammation. Some compounds' low solubility concentrations can be addressed by using additional dissolution techniques, as suggested by Falade, et al., [33]. The AMES toxicity prediction and lipinski drug-like test results indicate that the drug candidates are safe for human consumption. None of the four qualified compounds were found to cross the blood-brain barrier, which means they are unlikely to cause neurological damage or addiction in patients. The findings of this study can be used to guide future research and development of these drug candidates for the treatment of diabetes, hypertension and inflammation.
In conclusion, the findings from this research were able to detect the anti-inflammatory, anti-hypertensive and anti-diabetic characteristics of AVL and AVS. The molecular docking simulation findings demonstrated that the binding energies of AVL compounds L01 (Stigmasta-5,22-dien-3-ol) and L02 (campestenol), as well as AVS compounds S02 (Ergost-5-en-3-ol) and S03 (Stigmastan-3,5,22-trien), exceeded those of conventional drugs (control drugs) used to treat diabetes, hypertension and inflammation. This implies that these bioactive compounds might be interesting drug candidates to look into in further drug development procedures. Furthermore, the findings suggest that the therapeutic efficacy of avocado varies, with inflammation having the most potential, followed by hypertension and diabetes. Additionally, AVL had a larger phenolic content, indicating greater antioxidant activity, but AVS had a higher flavonoid concentration, indicating greater total antioxidant and health-promoting qualities. Our findings shines light on the phytochemical profile and molecular docking studies of AVL and AVS, indicating their potential antioxidant action and health advantages.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ademoyegun OT, Ahmed RS, Raphael DO, Mustapha BO, Salau S. Phytochemical Profiling and Molecular Docking Investigation of Avocado (Persea americana mill. Cultivar Hass) Leaves and Seeds: Implications for Antioxidant Activity and Health Benefits. J Agri Sci and Food Res. 15:168.
Received: 22-Jan-2024, Manuscript No. JBFBP-24-29263; Editor assigned: 24-Jan-2024, Pre QC No. JBFBP-24-29263 (PQ); Reviewed: 07-Feb-2024, QC No. JBFBP-24-29263; Revised: 14-Feb-2024, Manuscript No. JBFBP-24-29263 (R); Published: 21-Feb-2024 , DOI: 10.35248/2593-9173.24.15.168
Copyright: © 2024 Ademoyegun OT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.