Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
+44 1300 500008
ISSN: 2167-0412
+44 1300 500008
Research Article - (2012) Volume 1, Issue 8
An increasingly detailed understanding of plant growth and development continues to emerge through a combination of experimental and analytical approaches. The present study includes a brief record of observations on the vegetative growth in the yam plant Dioscorea deltoidea, in the neighborhood of its procurement place-Dachigam National Park, Kashmir. The plant adapted quite well at the comparatively lower elevation, allowing the reasonable measurements to be made, and providing a spark of hope for its use, as an economical species, as well as serving as a point forward for its conservation.
Keywords: Plant growth; Growth rate; Yam; Dioscorea deltoidea; Steroids; Diosgenin; Ethnobotany
All seed plants pass through three stages during the development of the sporophyte: embryogenesis, vegetative development, and reproductive development. With germination, the embryo breaks its dormant state, and by mobilizing stored reserves, the seedling commences a period of vegetative growth. Depending on the species, germination can depend on a variety of factors, including moisture levels, extended cold, heat, and light. Drawing initially on reserves stored in its cotyledons (e.g. beans) or in endosperm (e.g. grasses), the plant builds on its rudimentary form, through the activity of the root and shoot apical meristems. Through photomorphogenesis and further development of the shoot, the seedling becomes photosynthetically competent, thus enabling further vegetative growth. After a period of vegetative growth, plants repond to a combination of intrinsic and extrinsic cues, including size, temperature and photoperiod, to undergo the transition to reproductive development. In flowering plants, this transition involves the formation of specialized floral meristems, that give rise to flowers [1].
Each of the three dominant phases in the life cycle of plants (establishment, growth and reproduction (seed-set)) has an impact on demographic stochasticity of the population. Similarly, environmental stochasticity also has an impact selectively at these three specific phases, and on the life cycle as a whole [2-5]. Among the three phases, the growth phase has been considered as the critical one influencing demographic stochasticity. Plants spend maximum duration in this phase. This increases the probability of exposure to shocks, thereby allowing for greater damages. The other two phases last for shorter durations than the growth phase, thereby minimizing the impact of shocks [6].
Ethnobotany of Dioscorea
The genus Dioscorea L., a monocotyledon, belonging to the family Dioscoreaceae, comprises 350-400 species [7], and is distributed throughout the tropics and subtropic regions, especially in West Africa, parts of Central America and the Caribbean, the Pacific Islands and Southeast Asia. Disocorea deltoidea has been much sought after by private agencies and pharmaceutical firms, having been continuously collected in India, except perhaps in the more inaccessible areas of the Himalayas [8,9]. Its natural range includes parts of Afghanistan, Pakistan, India, Nepal, Bhutan, China and Vietnam (Boxes 1-3). The roots yield cortisone, a steroidal hormone, used in treating rheumatic diseases and ophthalmic disorders.
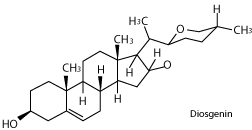
The genus has recently gained much repute as a source of steroidal sapogenins like diosgenin. These are promising starting material for synthesis of cortisone, which is useful in treatment of rheumatic arthritis, and in preparation of sex hormones [10]. Diosgenin is a precursor for the chemical synthesis of steroidal drugs, and is tremendously important to the pharmaceutical industry [11].
The evolving commercial importance of the secondary metabolites has in recent years resulted in a great interest, in secondary metabolism, and particularly in the possibility to alter the production of bioactive metabolites, by means of cell culture technology. Plant cell culture systems represent a potential renewable source of valuable medicinal compounds, flavours, fragrances, and colorants, which cannot be produced by microbial cells or chemical synthesis [12]. The search for high-producing cell lines coupled to recent developments in immobilized cultures and the use of extraction procedures, which convert furostanol saponins to spirostanes such as diosgenin, should prove useful in increasing productivity in the years to come [13]. The establishment of suspension cell culture for the production of secondary metabolite, diosgenin, using 2,4-D single treatment has been reported in Dioscorea deltoidea [14]. Dioscorea deltoidea suspension cultures are capable of metabolizing progesterone to 5α-pregnan-3β- ol-20-one and 5α-pregnan-3β,20β-diol [15]. Stohs [16] found that the microsomes isolated from Cheiranthus cheiri and Dioscorea deltoidea converted progesterone into a single metabolite, 5α-pregnane-3,20- dione, in the presence of an NADPH-regenerating system; with an optimum pH of about 7 during the reaction. The aglycon form of the steroidal sapogenin furost-5-ene-3β,22,26-triol, 3β-chacotrioside 26β-D-glucopyranoside (furostanol I), isolated from cell suspension cultures of Dioscorea deltoidea and its molecular structure determined by mass spectrometry and ‘H and 13C n.m.r. spectroscopy; from kinetic studies and incorporation experiments with [1-14]acetate, it has been inferred that the steroidal compound (in the glycoside form) is an intermediate in vivo in diosgenin biosynthesis. It accumulated in growing cells of D. deltoidea and was metabolized to diosgenin (in the glycoside form, i.e. dioscin) in non-dividing cells [17].
A new steroidal saponin, orbiculatoside B, together with a pair of furostanol saponins, protobioside and methyl protobioside, from the fresh rhizomes of Dioscorea deltoidea Wall var. orbiculata was isolated via bioactivity-guided fractionation, the compound being identified as isonarthogenin 3-O-α-L-rhamnopyranosyl(1→2)-[α- L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside, by various NMR techniques in combination with chemical methods; the three saponins showing strong activity against Pyricularia oryzae, and being cytotoxic to cancer cell line K562, HCT-15, A549, and A2780a in vitro [19].
Yams also serve as a common article of food, particularly among the hill tribes and the poor. Some species of Dioscorea yield industrial starch [10]. Dioscorea is the main source of carbohydrate for the Sakai tribe at Banthad Range, Peninsular Thailand [20,21].
The ethnobotanical explorations conducted during 1982-92 by Kaul [22], have depicted that vast storehouses of herbal charms still exist with the hill tribes living peacefully in the far-flung mountain ranges of J&K.
Little regard has been given to members of the plant kingdom, which are potentially or actually useful in traditional medicine, but whose members may be declining rapidly due to habitat destruction or over-exploitation [23]. It is estimated that 70–80% of people worldwide rely chiefly on traditional, largely herbal medicine to meet their primary healthcare needs [24,25]. Kashmir has a long history of utilization of herbal drugs. There has been a continuously growing tradition of herbal treatment, and both Ayurvedic and Unani systems of medicine have played a major in the health care systems of this region [22].
All CITES Appendix I and Appendix II plant species obtained from the wild is prohibited for export from India. Only cultivated/ artificially propagated plant species listed under App. II is allowed for export under cover of CITES export permit and Legal Procurement Certificate (L.P.C.), or certificate of cultivation from the designated authorities. Export of Dioscorea deltoidea requires ‘certificate of cultivation’ or Legal Procurement Certificate’ from the designated authorities of the Forest Department, as per MOEF circular dt. 4.10.2000. According to the Export and Import Policy 1997-2002, Schedule 2-Appendix 2, the export of Dioscorea deltoidea (Elephant’s foot) plants, plant portions and their derivatives, and extracts as such obtained from the wild, except the formulations made there from, is prohibited.
The 15th CITES Conference of the Parties Meeting (CITES CoP15), March 13 to 25, 2010 at Doha, Qatar, provided the occasion for Parties to CITES to Review of Significant Trade in specimens of Appendix- II plant species. Two draft decisions regarding Dioscorea deltoidea, Cistanche deserticola, Nardostachys grandiflora, Picrorhiza kurrooa, Pterocarpus santalinus, Rauvolfia serpentina and Taxus wallichiana were accepted by consensus, as per below and here:
‘Directed to the range States of Dioscorea deltoidea, Cistanche deserticola, Nardostachys grandiflora, Picrorhiza kurrooa, Pterocarpus santalinus, Rauvolfia serpentinaand Taxus wallichiana, to the regional representatives for Asia on the Plants Committee and to the Secretariat, that the bodies to which this Decision is directed should ensure the implementation of regionally coordinated actions, to improve the management of the seven species and ensure that the trade therein is legal, sustainable and traceable. These measures could include, inter alia, the organization of regional capacity-building workshops, the improvement of methodologies to make non-detriment findings, and to determine legal acquisition, the harmonization of management and compliance measures, and the development of incentives to prevent illegal trade. Directed to the Secretariat that The Secretariat shall: a) subject to the availability of external funding and in collaboration with the range States, the regional representatives for Asia on the Plants Committee, the World Health Organization, traditional medicine associations and TRAFFIC, organize one or several regional capacitybuilding workshops, on the basis, inter alia, of the recommendations in document PC17 Inf. 10; and b) inform the Plants Committee on progress made at its 20th meeting’ (Report from CITES 15th Conference of the Parties Meeting March 13-25, 2010, IUCN).
The species, Dioscorea deltoidea, can be easily propagated by underground corms or aerial bulbils. Vegetatively propagated plants have relatively faster growth than the seedlings; hence it is the preferred method [26]. Dioscorea deltoidea advertises an immense potential in the pharmaceutical industry, as well in the traditional medicine. However, demand on the wild stocks imperils the future survival of this important medicinal plant. Thus, in order to conserve the species, as well as to harness the potential economic benefits to the local people, the awareness regarding the easeful growing of these plants need to be highlighted and provision of yam tubers need to be established by the concerned authorities of the state, to the buffering areas of mountains. The plants also have an appealing growth pattern. The plants grow quite beautifully, giving a pleasing aesthetic look to the garden.
The material for growing the Dioscorea deltoidea plants (Figure 1) comprised the underground corms or tubers collected from the Dachigam National Park, Srinagar (Kashmir, J&K), from an elevation of about 2500 m. The plants were grown in the neighborhood area situated about 12 Kms from the National Park, at an elevation of about 1595 m. The plants were provided the support of the 6 mm iron rods. The plants were allowed to grow again and again for two generations, and the observations on the growth of the plants were recorded during the third generation of their growth. The plants were found to grow very well each year, however, failed to produce the seed set, as such the growth was continued every year by the plants from the corms.
Figure 1: Growing plants of Dioscorea deltoidea*.
*The study plants were labeled simultaneously, however, they were differing in their time of emergence from few days to a week at the time when the first measurements were recorded. Owing to this, the measurements were noted as Day 1, Day 2, and so on.
The length of the plants was measured with the help of a standard tape. Since the plants twine around the support, the measurements were taken directly lengthwise, assuming the twining to be constant around the iron rod and lesser in magnitude to affect the measurements. Different plants started germination at different times, being separated by a difference, ranging from few days to a week or more, and therefore, this helped in clubbing the growth rate of the different plants to reflect a continuous growth pattern.
The growth of the yam plants as observed during this study are complied in table 1. The plant growth measurements were carried out on the plants after their germination, however, some plants germinated at a comparatively different time, lagging or leading by few days to a week or more. This helped in combining the plant growth at different intervals to fairly extrapolate it into a continuous measurement, for depicting the growth in the plants. The growth rate appeared to be slower during the first two weeks (<1 cm per day), and then progressed at a relatively faster rate during the third week. The growth rate was quite high from the fourth week (2-4 cm per day), and significantly higher from the fifth week onwards (ranging from 4-8 cm per day) (Figure 2 and 3).
| Dioscorea deltoidea | Plant 1 | Plant 2 | Plant 3 | Plant 4 | Plant 5 | Plant 6 | Plant 7 | Plant 8 | Plant 9 |
|---|---|---|---|---|---|---|---|---|---|
| DAY 1* (April 3, 2009) | 1.40 | - | 6.20 | 11.10 | 11.30 | 14.60 | 26.80 | 28.00 | 36.00 |
| DAY 3 (April 5, 2009) | 1.80 | 2.00 | 7.70 | 15.90 | 13.40 | 20.10 | 40.00 | 37.50 | 59.00 |
| DAY 5 (April 7, 2009) | 2.60 | 4.40 | 9.90 | 20.50 | 16.40 | 25.00 | 51.80 | 45.20 | 74.50 |
| DAY 8 (April 10, 2009) | 4.00 | 3.10 | 12.00 | 26.00 | 20.00 | 32.00 | 63.00 | 56.00 | 86.50 |
| DAY 11 (April 13, 2009) | 6.50 | - | 18.00 | 39.00 | 26.00 | 48.00 | 93.00 | 82.00 | 115.00 |
| DAY 17 (April 19, 2009) | 20.50 | - | 44.00 | 99.00 | 30.50 | 111.00 | 174.00 | 152.00 | 183.00 |
| Average growth per day, cm ▼ | |||||||||
| DAY 1 – DAY 3 | 0.13 | - | 0.50 | 1.60 | 0.70 | 1.83 | 4.40 | 3.16 | 7.66 |
| DAY 3 – DAY 5 | 0.26 | 0.13 | 0.73 | 1.53 | 1.00 | 1.63 | 3.93 | 2.56 | 5.16 |
| DAY 5 – DAY 8 | 0.35 | 0.23 | 0.52 | 1.37 | 0.90 | 1.75 | 2.80 | 2.70 | 3.00 |
| DAY 8 – DAY 11 | 0.62 | - | 1.50 | 3.25 | 1.50 | 4.00 | 7.50 | 6.50 | 7.12 |
| DAY 11 – DAY 17 | 2.00 | - | 3.71 | 8.57 | 0.64 | 9.00 | 11.57 | 10.00 | 9.71 |
Table 1: Growth of Yam plants (Dioscorea deltoidea) recorded at different times during the growing phase.
This mini study highlights the general pattern of growth in vegetation. From germination till the adjustments by the newly emerged plants, the growth is somewhat slower, but after the successful adjustment in the surrounding environment, the growth is progressed onwards at a higher rate. The principles and the underlying philosophy of plant growth can be related by the already established ecological facts of the plant growth, and thus the same has been provided ahead.
The vegetative phase of development begins with embryogenesis, which initiates plant development. Embryogenesis establishes the basic plant body plan and forms the meristems that generate additional organs in the adult. Plant development is an ongoing process; the vegetative meristems are highly repetitive-they produce the same or similar structures over and over again-and their activity can continue indefinitely, a phenomenon known as indeterminate growth. When the adult plant undergoes a transition from vegetative to reproductive development, culminating in the production of a zygote, the process begins again. At some point in the life cycle, the adult plant undergoes senescence and dies, and this too, is a developmentally controlled, genetically determined process [1].
An essential aspect of almost all land plants is their sedentary lifestyle. By virtue of their ability to photosynthesize, favorably positioned plants can readily obtain both the energy and the nutrients they need to grow and survive. Because plants are unable to relocate to optimal habitats, they must instead adapt to their local environments. While this adaptation can occur on a physiological level, it may also be achieved through a flexible pattern of development. Plants elaborate their forms throughout their lives through programs of vegetative development [27]. Through the regulated proliferation of undetermined cells of meristems and their recruitment into tissues and organs, plants are able to produce complex, but often variable forms that are best adapted to their local environment. The three-dimensional architecture of the shoot and root systems of most plants is elaborated gradually throughout the life of the individual. In addition to vertical growth by the axes (stem and root), lateral organs are produced. Shoots differ from roots in giving rise to leaves, but both roots and shoots exhibit branching patterns. Although plant growth can be influenced by many environmental factors, three main guidance systems control the orientation of the plant axis–phototropism, gravitotropism and thigmotropism. Thigmotropism, or growth with respect to touch, enables roots to grow around obstacles, and is responsible for the ability of the shoots of climbing plants to wrap around other structures for support [1].
Light serves both as a source of energy and as a source of information for green plants. It is a source of energy for photosynthesis, and a source of information for photoperiodism (night/day length), phototropism (light direction), and photomorphogenesis (light quantity and quality). The sensitivity of plants to light provides them with the sense of vision, although a very different kind of vision than that provided by the eyes of humans and animals [28]. Both chlorophylls and phytochrome absorb blue light (400-500 nm) from the visible spectrum, and other chromophores and some amino acids like tryptophan, absorb light in the ultraviolet (250-400 nm) region. However, phytochrome plays important ecological roles for plants growing in the environment. Phytochrome is involved in regulating various daily rhythms, plant sense, and response to shading.
German botanist Julius von Sachs (1832-1897) proposed that chemical messengers (hormones) are responsible for the formation and growth of different plant organs. Whereas other plant hormones seem to act as on/off switches that regulate specific developmental processes, auxin and cytokinin appear to be required at some level, more or less continuously. The levels and distribution of auxin affect a variety of processes, including the establishment of apical-basal polarity and process of organogenesis. Polarized auxin movement is essential to the development of the basic shoot-root polarity of the plant. The presence alone of auxin is not sufficient; a polarized flow of auxin through the developing tissues is also necessary. Regulation of growth in plants may depend in part on the amount of free auxin present in plant cells, tissues, and organs. There are two main pools of auxin in the cell: the cytosol and the chloroplasts. One of the most important roles of auxin in higher plants is the regulation of elongation growth in young stems and coleoptiles. The ability of protons (one of the important actions of auxin is to induce cells to transport protons) to cause cell wall loosening is mediated by a class of proteins called expansions, which loosen the cell wall by breaking hydrogen bonds between the polysaccharide components of the wall. Gibberellins affect many aspects of plant growth and development, although they are best known for their dramatic effects on internode elongation in grasses and dwarf and rosette species. Other physiological effects include roles in determination of flower sex, promotion of fruit growth, and germination of seed. The growthpromoting effects of BRs (Brassinosteroids were originally discovered as growth-promoting substances isolated from pollen) are reflected in acceleration of both cell elongation and cell division, although the major effect is on cell expansion. The process of cell expansion involves cell wall relaxation, followed by the osmotic transport of water into the cell to maintain turgor pressure, and cell wall synthesis to maintain wall thickness. As BRs are known to stimulate cell expansion and division, it is likely that BRs facilitate germination by stimulating the growth of the embryo [1].
Multicellular plants rely on growth in localized regions that contain small, undifferentiated cells, and may be many millimeters from the nearest differentiated xylem and phloem, water and solutes must move to these small cells for their growth. Increasing evidence indicates that after exiting the xylem and phloem, water and solutes are driven to the growing cells by gradients in water potential and solute potential, or concentration. The gradients are much steeper than in the vascular transport system and can change in magnitude, or suffer local disruption with immediate consequences for growth [29]. The enlargement of plant organs involves uptake of water by the cells and expansion of the cell walls, because of the resulting turgor pressure (internal hydrostatic pressure). Nutrient and metabolite deposition accompany this process, causing the water uptake that creates the pressure and sustains the enlargement. For land plants, a vascular system carries these substrates for long distances, but growing cells often are located several cell lengths, and possibly several millimeters, from the nearest xylem or phloem element. After leaving the vascular system, the movement occurs through tissues anatomically unmodified for transport, but this last section of the path is an essential part of the supply chain for growth. Because both solutes and water are involved, factors affecting the movement of either type of molecule will alter the growth of the cells [29]. There is substantial evidence that water moves from cell to cell, driven by gradients in water potential or its components [30]. Potential gradients indicate the change in potential per unit distance and in the xylem; the gradients consist mostly of tensions (negative pressure per unit distance along the xylem). In the phloem, bulk-solute flow is driven long distances by positive hydrostatic pressure gradients. Where water must cross the membranes, it is the sum of osmotic and hydrostatic potentials, i.e. the water potential that drives the flow [30]. Therefore, outside of the xylem and phloem in growing tissues, the implication is that gradients in potential should also exist [29]. These properties produce several consequences, perhaps the most obvious being that growing tissues must have turgor pressures low enough to form the required growth-sustaining Ψw gradients, but high enough to enlarge the cells. Factors that alter the rate of growth thus affect the steepness of the gradient. Examples, such as decreasing growth with cold [31], auxin deprivation [32] or cell maturation [33] cause turgor to increase, when the growth-sustaining gradients decrease [29].
Plant roots may grow continuously throughout the year. Their proliferation, however, depends on the availability of water and minerals in the immediate microenvironment surrounding the root, the so-called rhizosphere. If the rhizosphere is poor in nutrients or too dry, root growth is slow. As rhizosphere conditions improve, root growth increases. When excess minerals are present in the soil, the soil is said to be saline, and plant growth may be restricted if these minerals reach levels that limit water availability, or exceed the adequate zone for a particular nutrient. pH is an important property of soils, because it affects the growth of plant roots and soil microorganisms [1].