
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Case Study - (2020)Volume 11, Issue 4
The aim of the work was to evaluate the role of maternal platelet glycoprotein IIb/IIIa polymorphism A2 in early recurrent pregnancy loss. Pregnancy loss (RPL) is one of the most frustrating and difficult areas in reproductive medicine because the etiology is often unknown and there are few evidence-based diagnostic and treatment strategies. The study was carried out on twenty-five non pregnant patients who had at least two consecutive early pregnancy losses with age ranging from twenty to thirty-seven years. The study also included a control group for the molecular part of the study of twenty-five non pregnant women.
Polymorphism; Recurrent pregnancy loss (RPL); Thrombophilia
Recurrent pregnancy loss (RPL) is one of the most frustrating and difficult areas in reproductive medicine because the etiology is often unknown and there are few evidence-based diagnostic and treatment strategies [1]. RPL is defined as two or more failed clinical pregnancies as documented by ultrasonography or histopathologic examination [2]. Approximately 15 percent of pregnant women experience sporadic loss of a clinically recognized pregnancy. Just 2 percent of pregnant women experience two consecutive pregnancy losses and only 0.4 to 1 percent has three consecutive pregnancy losses [3]. Couples who have had a pregnancy loss have two major concerns: the cause and the risk of recurrence. Unfortunately, the cause of RPL can be determined in only 50 percent of patients [4]. General etiological categories of RPL include anatomic, immunological, genetic, endocrine, thrombophilia, and environmental factors. Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis [5,6]. A significant proportion of the population has a detectable abnormality in blood, but most of these only develop thrombosis in the presence of an additional risk factor [6]. The human glycoprotein (GP)IIb/IIIa is one of the best characterized receptors present on the surface of platelets [7]. GPIIb/IIIa belongs to the large family of adhesion molecules called integrins, which share a common heterodimeric structure. The primary function of GPIIb/IIIa is to aid platelet aggregation [8]. Nearly 20 years ago, Savage et al. demonstrated that the GPIIb/IIIa on the membrane of nonactivated platelets serves as a specific receptor for surfacebound fibrinogenbut, after platelet activation this receptor acquires the ability to interact with other adhesive proteins, such as vitronectin, fibronectin and von Willebrand factor [9,10]. GP IIb/IIIa, like other glycoproteins of the platelet membrane, is highly polymorphic, and Polymorphism A is the most frequently involved in immune-mediated platelet destruction. The molecular basis of this polymorphism is a T-to- C change at position 1565 in exon 2 of the GP IIIa gene. As a result, PlA1 molecules have a leucine, whereas PlA2 proteins have a proline, at position 33 of the mature β3 integrin chain [11]. The Leu33Pro amino acid substitution is likely to result in significant conformational changes in the structure of the GPIIb/IIIa receptor, as inferred from the nature of the amino acids involved. In fact, preliminary evidence seems toindicate that the PlA2 polymorphism conveys increased platelet agreeability [12].
The aim of the work was to evaluate the role of maternal platelet glycoprotein IIb/IIIa polymorphism A2 in early recurrent pregnancy loss.
The study was carried out on twenty five non pregnant patients who had at least two consecutive early pregnancy losses with age ranging from twenty to thirty seven years. The study also included a control group for the molecular part of the study of twenty five non pregnant women. They were with matched age with uncomplicated pregnancy and at least one child and no history of fetal loss. For cases we excluded other causes of early RPL e.g. genetic, immune, endocrinal, infectious, and anatomical and other thrombophilic causes of first trimester RPL.
All the cases provided signed informed consent for their admission to the study. The cases were subjected to the following: Careful history taking (Personal, Obstetrical and gynecological, Medical history of chronic diseases and Family history of thrombosis or RPL), General and local examination and Pelvic ultrasound examination.
Investigations to exclude other causes of RPL (TSH, Serum prolactin, Detection of lupus anticoagulant and anticardiolipin antibodies, Factor V Leiden mutation, Antinuclear antibodies (ANA) and Karyotyping for cases and their husbands).
Genomic DNA was isolated from whole blood by GeneJet Whole Blood Genomic DNA Purification Mini Kit (Thermo Scientific, Lithuania) using whole blood genomic DNA purification main protocol. DNA concentration of each case was measured on the Nanodrop (Spectrophotometer ND-1000) for quality and quantity. PCR Amplification was achieved by Dream Taq Green PCR Master Mix (2X) (Thermo Scientific, Lithuania) and primers used were: Forward primer (SENSE- 9): 5 ’ d CTTCTGACTCAAGTCCTAACG 3 ’ and Reverse primer (ANTISENSE -9): 5 ’ dCTTCTGACTCAAGTCCTAACG 3 ’ using thermocyclar (QB-96). The thermal cycling conditions are summarized in Table 1. Digestion of PCR product by restriction enzyme "FastDigest MSPI" (Thermo scientific, Lithuania) was performed and the digest was loaded into the QIAxcel instrument. Detection of restriction bands by gene capillary electrophoresis using QIAxcel DNA High Resolution Kit (Qiagen, Germany). The various genotypes were recognized according to the presence of the bands and the band length: PLA1/A1 results in 2 bands (279 bp and 197 bp), PLA2/A2 in 2 bands (173 bp and 106 bp) and PLA1/A2 results in 4 bands (279 bp, 197 bp, 173 bp and 106 bp) (as shown in Figure 1).
| Step | Temperature | Time | Number of cycles |
|---|---|---|---|
| Initial denaturation | 95 °C | 1-3 min | 1 |
| Denaturation | 95 °C | 30 s | |
| Annealing | 61 °C | 30 s | 25-40 |
| Automated fluorescent extension | 72 °C | 1 min/kb | |
| Final extension | 72 °C | 5-15 min | 1 |
Table 1: Thermal cycling conditions using thermocyclar (QB-96).
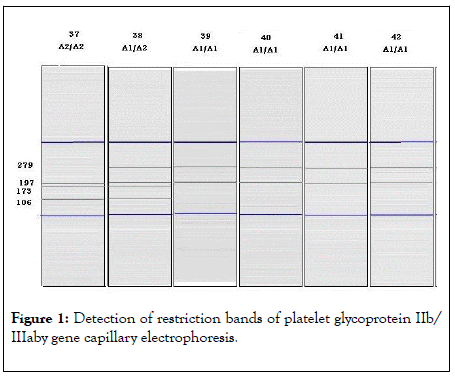
Figure 1: Detection of restriction bands of platelet glycoprotein IIb/ IIIaby gene capillary electrophoresis.
Baseline characteristics of the patients
The study population (n=50) consisted of 25 cases and 25 control. There were no statistically significant differences in baseline clinical characteristics between both groups (Age, medical history, family history and pelvic ultrasound examination). There were no statistically significant differences in laboratory tests (TSH, Serum prolactin, lupus anticoagulant, anticardiolipin, antinuclear antibodies and Factor V leiden mutation).
Frequencies of genotypes and alleles
Three groups of cases and control were determined according to their genotypes frequency (80% had the A1A1, 10% had the A1A2 and 10% had the A2A2 genotype).Twenty one of cases had the A1A1 genotype (84%) compared to nineteen (76%) of the control group. Two cases had A1A2 genotype (8%) compared to three (12%) of the control group. Two cases had A2A2 genotype (8%) compared to three (12%) of the control group and there were no statistically significant differences between both groups according to their genotype frequencies (Table 2 and Figure 1). Fourty four A1 alleles (88.00%) were found in cases compared to fourty one (82.00%) in the control group. Six A2 alleles (12.00%) were found in cases compared to nine A2 alleles (18.00%) %) in the control group. The prevalence of A2 allele was increased in the control group (18.00%) than cases (12.00%) but to a non-significant level (Table 3 and Figure 2). According to Hardy Weinberg equation the observed values for the three genotypes frequencies in cases demonstrated significant differences in comparison (Figure 3) with the corresponding expected values (p=0.008) and for the control group the observed values exhibited no significant differences compared to the corresponding expected values (p=0.0122) as shown in Table 4.
| Cases (n=25) | Control (n=25) | Total (n=50) | |
|---|---|---|---|
| Genotype | |||
| A2A2(mutant type) | 21 (84.00%) | 19 (76.00%) | 40 (80.00%) |
| A2A2(mutant type) | 2 (8.00%) | 3 (12.00%) | 5 (10.00%) |
| A2A2(mutant type) | 2 (8.00%) | 3 (12.00%) | 5 (10.00%) |
| Pearson Chi-Square | X2(df=2)=0.500 | ||
| p(MC)=0.778 NS | |||
Table 2: Comparison between cases and control according to their genotypes frequency.
| Cases (n=25) | Control (n=25) | Total (n=50) | |
|---|---|---|---|
| Genotype | |||
| A1 allele | 44(88.00%) | 41(82.00%) | 85(85.00%) |
| A2 allele | 6 (12.00%) | 9(18.00%) | 15(15.00%) |
| Pearson Chi-Square | X2(df=2)=0.710 | ||
| p=0.400 NS | |||
Abbrevations: NS: Statistically not significant (p>0.05); df: degree of freedom
Table 3: Comparison between cases and control according to glycoprotein IIb/IIIa Allele frequency.
| Groups | Observed | Expected | Pearson Chi- Square | ||||
|---|---|---|---|---|---|---|---|
| A1A1 | A1A2 | A2A2 | A1A1 | A1A2 | A2A2 | ||
| Cases (n =25) | 21 | 2 | 2 | 19.36 | 5.28 | 0.36 | X2(df=2)=9.65 |
| p=0.008* | |||||||
| Control (n = 25) | 19 | 3 | 3 | 16.8 | 7.38 | 0.81 | X2(df=2)=8.81 |
| p=0.0122 NS | |||||||
Abbrevations: NS: Statistically not significant (p>0.05); df: Degree of freedom.
* : Statistically significant (p<0.05)
Table 4: Comparison between cases and control according to their observed and expected frequencies of the various genotypes:
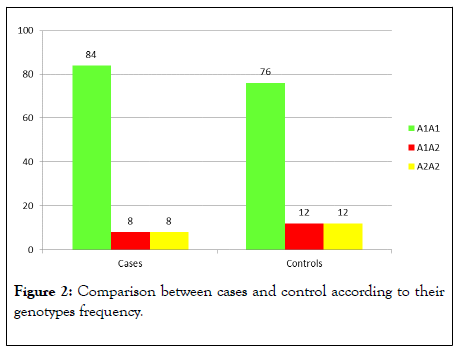
Figure 2: Comparison between cases and control according to their genotypes frequency.
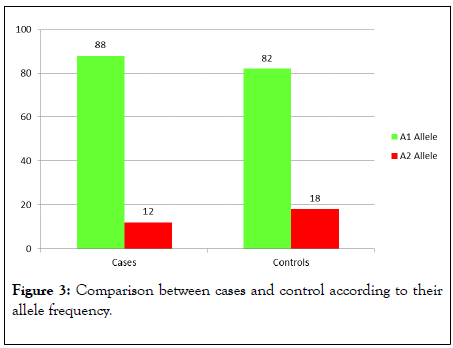
Figure 3: Comparison between cases and control according to their allele frequency.
Several studies in different countries among the world were designed to explore the role of inherited thrombophilia and RPL [13-15]. This current research studied the frequency of platelet glycoprotein IIb/IIIa polymorphism A2 in women with RPL.
Some researchers suggested that RPL is a multifactorial/ polygenetic condition, and several of the risk factors are insufficient independently for leading to RPL. It is when several intrinsic and extrinsic factors come together in the same individual that the risk exceeds the threshold and a thrombotic disorder leading to miscarriage [16].
The platelet glycoprotein IIb/IIIa receptor (GP IIb/IIIa) is the most abundant integrin on the platelet surface, and it has a central role in platelet aggregation, although it is also involved in platelet adhesion, the initial step in homeostasis.
Binding of fibrinogen to GP IIb/IIIa is the principal mechanism for platelet aggregation, while interaction with von Willebrand factor seems to be critical for platelet aggregation under high shear conditions. The clinical impact of PLA2 polymorphism has been investigated in several diseases, in which thrombus formation is a key pathogenetic factor.
Studies on this structural polymorphism have been carried on several pathologies [12,17]. As accepted Prothrombotic changes and thrombosis may interfere with implantation and placentation processes leading to miscarriage [18].
The present study reported that the prevalence of platelet glycoprotein IIb/IIIa polymorphism A2 is higher in control (18%) than cases (12%) but the difference was statistically nonsignificant (p=0.480).
Different results were obtained in similar studies in showing significant increase in prevalence of platelet glycoprotein IIb/ IIIa polymorphism A2 in cases than control e.g. Ivanov et al (2007)(19) found that 26.1% of cases compared to 12.5% of control (p=0.033) and Ivanov et al (2008)(7) also found significant prevalence in cases (p<0.0001) carriers of platelet glycoprotein IIb/IIIa PLA2 only, and when combined with other thrombophilic factors (PLA2 and FVL or PLA2 and FII G20210A) the risk was still high (p=0.058), but not significant.
Our results may be explained by the fact that first trimester fetal losses are caused by implantation failure (most are caused by cytogenetic abnormalities) rather than thrombotic tendencies [19,20].
Another accepted explanation is that impaired placental perfusion caused by inherited thrombophilia might not be critical for embryonic development during very early gestation due to very favorable placental/ embryonic ratio at this stage [21].
The present study focused on women with RPL, but it is likely that hereditary thrombophilia are also cause of isolated pregnancy losses. Carriers of these mutations exhibit highly variable phenotypic expression with most having no clinically noticed abnormalities. With respect to pregnancy losses, only a fraction of carriers will develop RPL, while others will experience no losses or possibly a single loss with an otherwise normal reproductive history. Since women who have a single loss are not commonly tested, and hereditary thrombophilia are usually clinically silent, the true cause of miscarriage thus remains undiagnosed. Clearly, the effects of thrombophilia on isolated pregnancy loss deserve further research.
The frequency of platelet glycoprotein IIb/IIIa polymorphism A2 is higher in the control group than women with early RPL but not to a significant level. This study did not find an obvious association between platelet glycoprotein IIb/IIIa polymorphism A2 and early RPL.
Citation: Sadek S, El Kaffash D, El Mahdy M, El Latif OA (2020) Platelet Glycoprotein iib/iiia (leu 33 pro) Polymorphism and its Role in Recurrent Early Pregnancy Loss. J Clin Cell Immunol.11:593. doi: 10.35248/2155-9899.20.11:593.
Received: 30-May-2020 Accepted: 12-Jun-2020 Published: 19-Jun-2020 , DOI: 10.35248/2155-9899.20.11.593
Copyright: © 2020 Sadek S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.