Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Review Article - (2013) Volume 2, Issue 3
Polyvinyl alcohol (PVOH) is a synthetic hydrophilic linear polymer that generally exists as a copolymer of vinyl alcohol and vinyl acetate. Therefore, the structural properties of PVOH polymers primarily depend on the degree of polymerization and the degree of hydrolysis, i.e., the ratio of the two monomers. Due to reactive functional groups on its structure, PVOH undergoes chemical changes such as esterification and etherification, as well as physical changes such as crystallization and ion-polymer complexation. Both chemically and physically-modified PVOH structures have found applications in biomedical and pharmaceutical area. This article reviews the properties of PVOH in solid and solution states, PVOH hydrogels and cryogels, their biomedical and pharmaceutical applications, and their potential challenges.
<Keywords: Polyvinyl alcohol; Hydrogel; Cryogel; Pharmaceutical use; Biomedical use
PVOH was first synthesized by Hermann and Haehnel in 1924 via saponification of poly(vinyl ester) in sodium hydroxide solution. Since vinyl alcohol is unstable and rapidly tautomerizes into acetaldehyde, the PVOH is commercially produced via hydrolysis of Poly(Vinyl Acetate) (PVAc) following a two-step process, i.e., free radical polymerization of vinyl acetate to PVAc followed by its hydrolysis. Structural properties of PVOH hence primarily depend on the molecular mass of the polymer and the degree of hydrolysis, i.e., the percentage of vinyl alcohol in the polymer [1,2].
The “n” in PVOH, -(C2H4O)n-, varies from 500 to 5000, which resembles change in molecular weight from about 20,000 to about 200,000 Daltons. Whether isotactic, syndiotactic or atactic, the PVOH tacticity is an important structural consideration that depends on the starting materials and the method of synthesis, and can be determined by NMR spectroscopy for instance. The PVOHs prepared from polymerization of vinyl acetate and its hydrolysis is atactic, whereas syndiotactic PVOHs can be prepared by radical polymerization of vinyl formate [3], vinyl pivalate, and vinyl trifluoroacetate. Isotactic PVOHs can be prepared by cationic polymerization of benzyl vinyl ether [4]. Properties offered by PVOH polymer are therefore associated with the method of preparation, molecular weight, tacticity, degree of polymerization and degree of hydrolysis. Properties like viscosity, resistance to solvents, adhesive strength, tensile strength, and film-forming are enhanced with increase in molecular weight and degree of hydrolysis [5]. On the other hand, glass transition and melting temperatures primarily depend on the degree of hydrolysis and tacticity [1]. PVOH polymers with no color and odor, melt at around 180-228°C, and display glass to rubber transition at 75-85°C. As the degree of vinyl acetate hydrolysis to vinyl alcohol increases, the polymer structure becomes more crystallized, which is associated with increased in intermolecular forces, melting and glass transition temperatures, and enhanced solubility in water [6].
Due to its crystallization, the PVOH structure is highly stable and chemically inert. It, however, undergoes reactions like any other secondary polyhydric alcohols [1]. PVOH undergoes esterification with both inorganic and organic compounds, reacts with boric acid and borax to form water insoluble cyclic esters [7], and goes into similar reactions with sulfur trioxide, alkane sulfonyl chlorides, titanium lactate, and titanium sulfate [5]. PVOH forms insoluble gels by reacting with poly(acrylic acid), and poly(methacrylic acid) [8] through interpolymer complexation. PVOH undergoes an internal etherification by losing water molecules in the presence of mineral acids or alkalis, and Michael’s addition with activated double bonds. PVOH can form intra-molecular and inter-molecular acetal compounds using different aldehydes, and can be chemically cross linked using difunctional aldehydes such as glutaraldehyde or glyoxal [9].
Due to its inertness and stability, PVOH is generally considered safe and biocompatible. PVOH is relatively safe when administered orally. The oral acute toxicity of PVOH (LD50) in rats and mice are 20 g/kg and 14.7 g/kg, respectively [10]. No data is available for LD50 values for inhalation and transdermal routes of administration. PVOH is poorly absorbed from gastrointestinal tract, and easily eliminated from the body. The 14C-labelled PVOH given orally to Fischer 344 rats as 0.01mg/kg single dose was found to be almost completely eliminated in the feces unchanged within 48 hours. In bioaccumulation study, similar results were observed and 0.05% of the total dose was detected in major tissues [11]. Sub-chronic toxicity and genotoxicity studies were conducted on Sprague-Dawley rats at doses of 2000, 3500 and 5000 mg/kg body weight/ day for a minimum of three months. With no adverse toxicity, the only major effect was the unformed stool with anogenital staining in rats at 3500 and 5000 mg/kg doses [12]. Below 10 w/v%, it didn’t cause irritation but caused anemia when injected subcutaneously [13].
Hydrogels are three dimensional cross linked hydrophilic polymers with the ability to absorb water or aqueous solutions into their structure [14,15]. Depending on the degree of chemical or physical cross linking, the amount of absorbed water varies as such they can be classified as low, medium, and high swelling hydrogels. PVOH hydrogels, due to their susceptibility to hydrogen bonding and excessive crystallization, generally offer very low swelling capacity, making them however very desirable for specific biomedical and pharmaceutical applications.
Formaldehyde, glutaraldehyde, and ketones are preferably used in the preparation of chemically-cross linked PVOH Hydrogels [16]. However, these are toxic, and the amount of residual cross linker left in the final polymer, and ways to reach to an acceptable and safe concentration can be quite challenging. Apparently, the molecular weight between the two crosslink points or crosslink density determines the final polymer properties such as the capacity of swelling, mechanical strength, rate of drug release, and its stability. However, such desirable properties are compromised by increased level of hydrogel toxicity and complicated purification at higher crosslinker concentration. Residual crosslinkers due to their functionality and reactivity have a strong potential to react with bioactive or drugs, and can alter the therapeutic properties of the final dosage form. Several studies have been conducted to evaluate the effect of crosslinking agents on drug diffusion, drug release and general properties of Hydrogels [17,18].
In order to resolve complications associated with the use of chemical crosslinkers, physical methods are preferred as PVOH polymers have the desirable functionality for physical crosslinking via irradiation or freezing-thawing, as well as ionotropic gelation. Hydrogels produced by irradiation offer better drug release properties than heat-cross linked Hydrogels [19]; they may however suffer from weak mechanical properties [16]. Nevertheless, as the name physical hydrogel implies, the hydrogel properties are generally reversible, and would compromise hydrogel stability.
Aqueous solutions of the PVOH polymer at high molecular weight (50,000-130,000 Daltons), high concentration (10-20 wt %), and high degree of hydrolysis (>98%) can successfully be cryogelled under freezing-thawing conditions. The hydroxyl groups (OHs) of the adjacent polymer chains will interact to form weak intra and intermolecular hydrogen bonds resulting in formation of crystallites [20,21]. The parameters affecting the cryogenic treatment are freezing/ thawing temperatures, number of freezing and thawing cycles, rate of freezing, and most importantly the thawing conditions [22]. Since PVOH solution in water is converted from liquid to a solid state during cryogelation, the composition of the polymer during such transition would determine the elastic, viscous and viscoelastic properties of these cryogels. For instance, similar to all other hydrogel systems, PVOH cryogels prepared at high polymer concentration or under the conditions enhancing mechanical properties, would display elastic properties while viscous properties would be favored under reverse conditions. In general, since the PVOH polymer and water are the only components of the hydrogel system, the cryogel property can be modulated to achieve desirable viscoelastic properties.
Urushizaki et al studied the degree of crosslinking, swelling kinetics, and viscoelastic properties of cryogels [23]. They found that the degree of cross linking and viscoelastic properties is increased with increase in number of freeze-thaw cycles. Moreover, the swelling kinetics of cryogels changed linearly with square root of time. Cryogels also displayed greater swelling in water at higher temperature [23]. Omidian et al. studied the use of PVOH cryogelation in enhancing mechanical properties of super porous hydrogels for gastric retention drug delivery applications. They conducted simultaneous polymerization chemical crosslinking of various hydrophilic monomers in the presence of PVOH solutions, followed by freezing thawing [24]. Xie et al. studied cryogel hybrids of PVOH and sodium alginate prepared via freezing thawing followed by calcium cross linking [25]. Mechanical properties of cryogels were evaluated by conducting comparative creep studies, no weight loss was observed and the strength of the gels was found dependent on the PVOH solution concentration, number of freezethaw cycles and freezing time [26]. The mucoadhesive properties of cryogels measured using a texture analyzer was decreased with increase in the number of freeze thaw cycles [27]. In another study, viscoelastic properties of the PVOH cryogels and PVOH ferrogels were studied [28]. In physical cryogels, the crystallites are thermodynamically unstable at higher temperatures, in other words crystallites will begin to melt at temperatures around the glass transition temperature of the polymer. Therefore, the thermal stability of the PVOH is desirable for applications where the service environment would experience a change in temperature. Thermal stability of cryogels can be enhanced, for example, by addition of co-solvents or stabilizers [20], or by adding thermo-resistant minerals. Like change in solution viscosity with time, the degree of crystallinity in cryogels is also expected to change with time, leading to change in mechanical property of the cryogel. Physical hydrogels such as PVOH cryogels are also susceptible to syneresis depending on the cryogelling conditions utilized during cryogel formation.
Using a texture analyzer with different accessories, Muppalaneni et al. studied adhesiveness, swelling, and viscoelastic properties of cryogels prepared at different polymer concentrations [29]. Adhesive properties were found to be dependent on the concentration of the polymer, and were favored at low polymer concentrations [30], whereas swelling of the cryogels prepared at higher polymer concentrations was found to be greater, and follow a diffusion-controlled mechanism [31].
Several articles studied the freeze thaw treatment of diluted and concentrated PVOH solutions, the effect of salt on swelling kinetics, and the structure/property relationship [32], as well as the mechanisms of PVOH cryotropic gelation. Few other studies evaluated the rheological and thermal properties, and sol-gel transition of the cryogel systems [33].
PVOH polymers can also be combined with different polymers to prepare composite hydrogels to improve properties like pH sensitivity, biocompatibility, drug release profile, etc. These include PVOH hydrogels in combination with poly(acrylic acid) [34], sodium alginate [25], locust bean gum [35], hydroxyethyl starch [36], gelatin [37], polyethylene glycol [38], and silkfibronin [39].
Due to their simple structure and unique properties such as adhesiveness, strength, film forming, biocompatibility, swelling, safety, and non-carcinogenicity, PVOH polymers have found applications in different industries including textile, paper, adhesives, food, biomedical and pharmaceutical in particular [32].
Biomedical applications
Properties such as high water content, elastic nature in the swollen state, biocompatibility, and swelling make the PVOH hydrogels a potential candidate as tissue replacement material. The PVOH hydrogels have been studied as soft contact lens material, artificial heart linings, artificial cartilages, catheters, skin, and pancreas membranes [32].
In early studies of PVOH hydrogels for biomedical applications, physical and gel properties were studied, and blood compatibility was the primary concern for the researchers. PVOH hydrogels prepared at low temperature crystallization, in particular, were studied as soft contact material, and displayed better optical properties, high oxygen permeation and low protein absorption than regular contact materials. Studies in rabbits found, however, no differences in the corneal epithelium [40,41]. Heparinization was carried out on hydrogels for blood compatibility and elastic properties [42]. PVOH hydrogels were prepared by low temperature crystallization followed by annealing were studied as artificial meniscus. Implanted PVOH hydrogels meniscus was reasonably good even after two years without any visible sign of wear or deformation [43].
PVOH cryogels were studied for articular cartilages, and their micro morphology was studied using differential scanning calorimetry and microscopy. Mechanical properties of the cryogels were also evaluated [44]. PVOH hydrogels implanted in bone, synovium, and muscle and their biocompatibility with tissues were studied histologically [45]. PVOH hydrogels were also studied for bioprosthetic heart valves [46], reconstruction of vocal cords [47], artificial kidney membranes for dialysis [48], and intervertebrate disc nuclei [49].
Pharmaceutical applications
Because of their biocompatibility, drug compatibility, watersolubility, film forming, good mechanical and swelling properties, the PVOH hydrogels have been studied as drug delivery systems in oral, transdermal, buccal, intramuscular, rectal routes of administration. Degree of crystallinity plays a major role in controlling diffusion of the drug from Hydrogels [16]. In general, PVOH hydrogels can be designed either as matrix or reservoir drug delivery platforms [50]. Altering gelling properties, solubility, adding copolymers have also been utilized to control the drug release from PVOH Hydrogels [51].
Mongia et al. evaluated the mucoadhesive properties of pure PVOH cryogels containing oxyprenolol and theophylline. They observed that mucoadhesive properties and drug release profiles would change with the number of freeze-thaw cycles [27]. Same group prepared PVOH hydrogels containing Ketanserin as a wound healing system, and studied its drug release profile and mucoadhesive properties [52]. They observed that cryogels undergone two cycles of freezing and thawing could have better adhesive properties, and release 80% of the drug in four hours. Ergotine tartrate buccal mucoadhesive dosage forms were formulated and evaluated for their adhesive properties, mechanical properties and drug release profile. The drug release slowed down at higher PVOH concentration [53].
PVOH hydrogels loaded with insulin prepared by emulsion polymerization followed by freezing thawing were studied in oral controlled drug delivery [54]. PVOH-locust bean gum hydrogels were prepared by emulsion chemical crosslinking method, and evaluated for the release of buflomedil hydrochloride. Hydrogels were evaluated for drug entrapment efficacy, particle size distribution, swelling properties, and drug release kinetics [35].
Bovine serum albumin was used as a model protein, and loaded into multi-laminate PVOH cryogels. Release of protein from cryogels undergone three or more freeze-thaw cycles was not significantly different. Zero order release was achieved by modifying different layers of cryogels [55]. Bovine serum albumin loaded PVOH cryogels were prepared by water in oil emulsion followed by freezing thawing. A nano-emulsion system was prepared by dispersing an aqueous solution of PVOH in oil using a homogenizer, followed by freezing thawing. The nano-cryogels were evaluated for particle size distribution, swelling, protein stability, and drug release. The protein was released following a diffusion-controlled mechanism that could be controlled by the number of freeze-thaw cycles, incubation temperature, and the degree of crystallinity [56]. PVOH cryogels loaded with fluconazole were evaluated for topical drug delivery, and compared to an oral route of administration. Polyethylene glycol was used as stabilizer, and evaluated for drug stability and release profile. Results suggested that cryogels were effective for topical drug delivery, and remained stable for 6 months [57].
The following graph shows the prevalence of the PVOH use in current pharmaceutical products as an excipient. As shown in the graph, PVOH polymers and copolymers are primarily used in manufacturing tablet dosage forms, followed by their use in ophthalmic, transdermal/ topical, and implant dosage forms [58]. Actual products containing PVOH homopolymer or copolymers are shown in Table 1.
| TABLETS |
|---|
| Trokendi XR (topiramate); Glumetza (metformin HCl); Khedezla (desvenlafaxine); Kombiglyze XR (saxagliptin and Metformin HCl); Nucynta ER (tapentadol); Oxtellar XR (oxcarbazepine); Pristiq (desvenlafaxine); Ranexa (ranolazine); Toviaz (fesoterodine fumarate); Ultram ER(tramadol HCl); Wellbutrin XL (bupropion hydrobromide); Gantanol (Sulfamethoxazole); Gralise (Gabapentin); Kaletra (lopinavir/ritonavir); Alinia (Nitazoxanide); Aplenzin (bupropion hydrobromide); Aricept (donepezil); Aromasin (exemestane); Atripla (efavirenz, emtricitabine and tenofovir); Azor (amlodipine and olmesartan medoxomil); Belviq (lorcaserin HCl); Bosulf (bosutinib); Caduet (amlodipine besylate and atorvastatin calcium); Dificid (fidaxomivin); Duexis (ibuprofen and famotidine); Iclusig (ponatinib); Incivek (telaprevir); Invokana (canagliflozin); Isentress (raltegravir); janumet (sitagliptin and metformin HCl); Januvia (sitagliptin); Juvisync (sitagliptin and simvastatin); kalydeco (Ivacaftor); Keppra XR (Levetiracetam); Letairis (Ambrisentam); Nucynta (Tapentadol); Oleptro (trazodone HCl); Onglyza (saxagliptin); Potiga (Ezogabine); Prezista(Darunavir); Savella (Milnacipran HCl); Selzentry (Maraviroc); Stivarga (Regorafenib); Stribild (elvitegravir, cobistat, emtricitabine, tenofovir DF); Teveten HCT (eprosartan mesylate, hydrochlorothiazide); Tivicay (Dolutegravir); Trecator (Ethionamide); Tribenzor (olmesartan medoxomil, amlodipine, hydrochlorothiazide); Tricor (Fenofibrate); Vibryd (vilazodone HCl); Vimpat (Lacosamide); Xarelto (Ricaroxaban) |
| IMPLANTS |
| Retisert (fluocinolone acetonide); Vitrasert (ganciclovir) |
| OPHTHALMICS |
| Bleph 10 (sulfacetamide sodium); genoptic (gentamicin sulphate); Ocufen (flurbiprofen sodium); FML (fluorometholone); HMS (medrysone); Poly-pred (prednisolone acetate, neomycin sulfate, polymyxin B sulfate); Pred-G (gentamicin sulfate and prednisolone acetate); |
| TRANSDERMAL/TOPICAL |
| Pliaglis (lidocaine and Tetracaine); Synera (lidocaine and Tetracaine); Ionsys (Fentanyl); Lidoderm (lidocaine) |
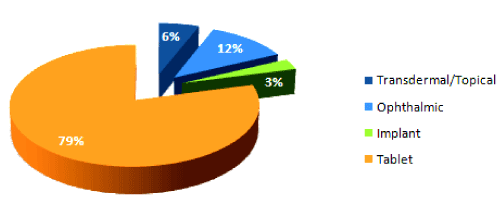 |
Table 1: Commercial pharmaceutical dosage forms containing PVOH polymers and copolymers [58].
Commercial suppliers of PVOH polymers and copolymers for industrial and pharmaceutical applications include EMD millipore (PVA Emprove, Ph Eur, USP is available in a variety of viscosities and grades of hydrolysis to suit various pharmaceutical applications and uses [59]), Sekisui Specialty Chemicals (Selvol), Dupont (Elvanol), Nippon Gohsei (Gohsenol), and Colorcon Inc. (Opadry grades, graft copolymers of ethylene glycol and vinyl alcohol). For instance, Selvol Ultralux FF is a high molecular weight PVOH homopolymer used for its film forming and adhesion promoting ability in body washes, multipurpose creams, and sun screens. The Selvol Ultralux AD is a copolymer of vinyl amine and vinyl alcohol with better surface activity and pH sensitive properties. The Selvol Ultralux SC is also a copolymer of vinyl pyrrolidone and vinyl alcohol for miscellaneous cosmetic applications [60].
PVOH polymers and copolymers can offer unique water retention, film forming, strength and swelling properties, which can be vitally beneficial in general health applications, and in particular pharmaceutical use. These have also found major application in biomedical filed due to non-toxicity and desirable swelling and mechanical property that they offer in their water-swollen states. However, the large scale manufacturing of this polymer and its copolymer for pharma application is currently very limited due to increased regulation for food and drug products. Moreover, the safety of the chemical cross linker residue in chemically-cross linked PVOHs, the thermodynamic stability of physical crystallites in physically-cross linked PVOHs, and stability of ion-cross linked physical PVOHs, drug hydrogel interaction, and long-term hydrogel stability are among very major challenges that require extensive and intensive research.