Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2013) Volume 1, Issue 2
Objectives: The pharmacokinetics of rifampin and its major metabolite desacetylrifampin after oral administration of rifampincapsules were studied in 24 healthy Middle Eastern Arab male volunteers. Middle Eastern Arabs were compared with Mexicans, Italians, Indians, Estonians, British and American Caucasians with respect to pharmacokinetic parameters of rifampin to seek evidence for polymorphism in rifampin pharmacokinetics. Methods: Each subject received a single dose of 600 mg (2×300 mg capsules) of rifampin after an overnight fast and plasma samples were drawn at specified times for a period up to 16 h after drug administration. Concentration of rifampin and its metabolie were determined using an accurate HPLC method. The data for other ethnic groups were extracted from published studies or solicited from investigators. Results: The maximum rifampin plasma concentration (Cmax) was 8.86 ± 2.74 μg/ml (Mean ± SD) and the time to reach maximum plasma concentration (Tmax) was 1.88 ± 1.12h.The maximum desacetylrifampin plasma concentration (Cmax,Met) averaged 0.96 ± 0.32 g/ml (Mean ± SD) and the time to peak plasma concentration of the metabolite (Tmax,Met) averaged 4.29 ± 1.3h.No statistically significant differences in most of rifampin pharmacokinetic parameters between Middle Eastern Arabs and other ethnic groups. Probit transformation of AUC data revealed a bimodal probit plot with breakpoint corresponding to an AUC of about 60 μg.h/ml. Conclusions: The data support the possibility of existence of interethnic differences in rifampin pharmacokinetics. American Caucasians, Tunisians and Middle Eastern Arabs could be grouped in one category, whereas Italians, Indians and Mexicans could be grouped in another category with respect to rifampin pharmacokinetics.
Keywords: Rifampin, Desacetylrifampin, Pharmacokinetics, Interethnic, Metabolism, Middle eastern arabs
Rifampin (INN, rifampicin), a semi-synthetic antibiotic derived from rifamycin B, is widely used in the treatment of tuberculosis and leprosy. It is also used for the prophylaxis of meningococcal disease and in the treatment of asymptomatic meningococcal carriers [1]. The drug inhibits the growth of most Gram-positive as well as many Gramnegative bacteria. Upon oral administration, rifampin is absorbed from the gastrointestinal tract and metabolized in the liver through deacetylation to produce 25-desacetylrifampin as the major metabolite [2,3].
It is well known that genetic background, age, environmental factors and disease state can affect the body’s ability to absorb, distribute and metabolize drugs. Differences in the metabolizing capacity of many drugs have been observed in many populations due to variations in genetic constitution. In recent years, the study of population variability in drug disposition and pharmacologic responsiveness has received increasing attention. The influence of interethnic and racial differences on pharmacokinetics and pharmacodynamics of drugs has been extensively reviewed in literature [4-13].
Humans can be characterized as poor or extensive metabolizers with use of racemic mephenytoin as a phenotyping drug, and CYP 2C19 has been identified as the major S-mephenytoin hydroxylase in humans [14- 17]. This polymorphism affects the disposition and pharmacokinetics of many clinically important drugs such as diazepam, omeprazole, propranolol, chloroguanide and imipramine [18]. Previous studies on drug metabolism have shown the possibility of existence of interethnic differences in metabolism of drugs such as nifedipine and omeprazole between Arabs and other ethnic groups [19,20]. A wide inter- and intra-individual variability in rifampin disposition was observed in many studies. This variability was attributed in part to formulation factors, age, sex, food and concomitantly administered drugs [21-30]. One report suggested the existence of interethnic differences in rifampin pharmacokinetics between Indonesians and Caucasians [31]. This claim was examined by other investigators who concluded that the higher plasma concentrations observed in the Indonesian study could have been due to the use of a generic rifampin formulation exhibiting fast dissolution leading to increased bioavailability [32]. In addition, they also recommended the use of an adequate reference formulation, such as Rifadin® since it is the most widely used formulation in pharmacokinetic studies.
The purpose of this study is to determine the pharmacokinetics of rifampin and desacetylrifampin in Middle Eastern Arabs living in Saudi Arabia in comparison to other Arab populations living in North Africa, namely, Tunisians. Furthermore, the results will be compared with those for other ethnic groups available in literatures to seek evidence, if any, for interethnic differences in rifampin pharmacokinetics. In addition, all previous studies were conducted using small sample sizes. The small sample size accompanied with the interindividual variability observed in rifampin pharmacokinetics could probably mask any significant difference between ethnic groups. The present study emphasizes the use of a fairly larger sample size to allow the detection of potential differences.
Subjects
Twenty-four healthy male adult volunteers participated in the study. Their mean age (± SD) was 34.3 ± 5.8 years with a range of 23 to 46 years, body weight of 73.4 ± 9.9 kg with a range of 58 to 90 kg and height of 171.3 ± 3.6 cm with a range of 165 to 179 cm. All subjects were within 10% of their estimated ideal body weight [33]. On the basis of medical history, clinical examination and laboratory investigation (hematology, blood biochemistry, and urine analysis), none of the subjects showed any evidence of hepatic, renal, gastrointestinal or hematologic deviations or any acute or chronic disease or drug allergy. The volunteers were asked to abstain from taking any drug including Over The Counter (OTC) for at least two weeks prior to and during the study. Informed consents were obtained from the subjects after explaining the nature and purpose of the study. All treatments were performed according to the guidelines of Helsinki Declaration for human research and its amendments, and the experimental protocols usedwere approved by King Khalid University Hospital (KKUH), College of Medicine Research Center (CMRC), King Saud University, Riyadh, Saudi Arabia.
Study protocol
Each subject received a single dose of 600 mg (2×300 mg capsules) of Rifampin (Rifadin®capsules, Lot No. 009, Gruppo Lepetit S.P.A., Milan, Italy) with 240 ml of water after an overnight fast for at least 10 hour. Beverages and food containing caffeine were not permitted over the entire course of the study. Volunteers were ambulatory during the study but were prohibited from strenuous activity. For a period of 16 hours following drug administration, the volunteers were under direct medical supervision in an outpatient clinic in King Khalid University Hospital.
Multiple venous blood samples (7 ml) were collected in evacuated heparinized glass tubes through an indwelling cannula placed in the forearm veins before (0 hour) and at 15, 30 min, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, 12.0, 14.0, 16.0 and 24.0 hours post-dose. The blood samples were immediately centrifuged at 3000 rpm for 10 min and the plasma decanted in coded polypropylene tubes containing 0.5 mg ascorbic acid per 1 ml plasma for maximum stability of rifampin and desacetylrifampin in plasma. The tubes were stored frozen at -70°C pending analysis.
Determination of rifampin and desacetylrifampin in plasma
An accurate, sensitive and reproducible reversed-phase High Performance Liquid Chromatographic (HPLC) method for the quantitation of rifampin and its main metabolite (desacetylrifampin) in plasmahas been used and validated. The chromatographic system consisted of solvent delivery pump (LC-10AD) and a variable UV detector (SPD 10-A) (Schimatzu, Kyoto, Japan) [34]. The procedure involved protein precipitation with acetonitrile. The drug and the internal standard p-nitropropionanilide were eluted from a 10-μmstainless steel μBondapak C18 column (3.9×300 mm) at room temperature with isocratic mobile phase consisting of 61% methanol in sodium phosphate buffer (pH 5.2), at a flow rate of 1.5 ml/min, and monitored in the UV detector at 337 nm. Each analysis required no longer than 16 min. The retention times were 4.7 min, 7.2 min and 15.5 min for the internal standard, desacetylrifampin and rifampin, respectively. Quantitation was achieved by measurement of peak height ratio of the drug and the metabolite to the internal standard. The limit of detection for rifampin and desacetylrifampin was 0.15 mg/l. The intraday coefficient of variation (CV%) ranged from 2.7% to 3.5% for rifampin and from 1.1% to 6.1% for desacetylrifampin at the concentration range evaluated. The interday CV ranged from 2.64% to 4.1% and from 2.47% to 5.25% at 0.8, 3, 6 and 12 mg/l for rifampin and at 0.4, 0.8, 3.0 and 6 mg/l (9 replicates for each concentration) for desacetylrifampin, respectively. The recoveries (absolute and relative) of rifampin and 25-desacetylrifampin from plasma were quantitated using four plasma standards. The relative analytical recovery was measured by adding the drug and the metabolite and the internal standard to drug-free plasma (12 replicates for each standard) to achieve the desired concentrations (0.8, 3.0, 6.0 and 12 mg/l for rifampin and 0.4, 0.8, 3.0 and 6.0 mg/l for 25-desacetylrifampin). The spiked plasma was then analyzed. The relative recovery was calculated by comparing the concentrations obtained from the drug-supplemented plasma with actual added amount. The absolute recovery was calculated by comparing the observed concentrations from the processed standard samples to direct injection of stock aqueous solution prepared at concentrations which represent 100% recovery. Relative recovery ranged from 100.22% to 105.43% and from 99.33% to 103.25% while the absolute recovery ranged from 97.78% to 102.76% and from 96.28% to 104.33% for rifampin and desacetylrifampin, respectively. Stability test shows that rifampin and its main metabolite desacetylrifampin are stable in plasma enriched with 0.5 mg ascorbic acid per each ml of plasma when stored at –70°C for a period of one month.
Pharmacokinetic analysis
The pharmacokinetic parameters for rifampin and desacetylrifampin were determined from the plasma concentrationtime data by noncompartmental analysis. The maximum plasma concentration (Cmax) and the time to reach maximum plasma concentration (Tmax) were obtained directly by inspection of the individual drug plasma concentration-time profiles, and were used as measures for the rate of absorption. The area under the plasma concentration-time curves up to the last time (t) showing a measurable concentration (Ct) of the analyte (AUC0-t) was determined by using the linear trapezoidal rule. The elimination rate constant (Kel) was calculated by least squares regression from the data of the last 4-6 points of each plasma concentration-time curve. The area under the plasma concentration-time curves up to infinite time (AUC0-∞) values were determined by adding the quotient of Ct and the appropriate Kel to the corresponding AUC0-t, that is:
AUC0-∞ =AUC0-t + Ct/ Kel
The elimination half-life (T1/2) of rifampin was also evaluated using the following equation:
t1/2 =ln(2) / Kel
The oral clearance (CL/F) was calculated asDose/AUC0-∞where F is the oral bioavailability. The mean residence time (MRT) was calculated using the following relationship:
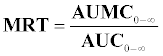
where AUMC0-∞ is the area under the first moment curve, and calculated by the trapezoidal rule from a plot of the product of rifampin plasma concentration and time vs. time. An estimate of the apparent volume of distribution at steady state (Vss) was obtained from the product of MRT and clearance.
The area under the curve to the last measurable concentration represented 96.5 ± 1.47% of the AUC0-∞ in Middle Eastern Arabs indicating the adequacy of sampling period. Data from published rifampin studies for other ethnic groups were obtained directly or extracted from the plasma concentration-time profiles of the respective studies by digitization. Also included in the analysis all studies that deal with rifampin pharmacokinetics in combination with other drugs if the results of these studies showed negative pharmacokinetic interaction with rifampin. Pharmacokinetic parameters obtained for doses other than 600 mg were normalized to 600mg dose assuming linear pharmacokinetics. If data for two different doses are available, the data for the higher dose were chosen.
Statistical analysis
Comparisons of pharmacokinetic parameters between Middle Eastern and other populations were carried out by the Student’s t-test for independent samples assuming homoscedastic or heteroscedastic model. The values ofTmaxwere compared using Wilcoxon rank sum test/Mann-Whitney “U” test since it has been reported that the distribution of this parameter does not follow a Gaussian distribution. Kolmogorov-Smirnov goodness-of-fit was applied to determine the normality of AUC distribution. The statistical level of significance was taken as 0.05 and results were expressed as mean ± SD with the 95% confidence interval and the actual p-value.
Rifampin was well tolerated by the subjects. There were no significant adverse events or protocol violations during the study. Unexpected incidents that could have influenced the outcome of the study did not occur. All the volunteers who started the study continued to the end and were discharged in good health. Rifampin was measurable at the first sampling time (0.25 h) in only two subjects following the administration of the drug, while plasma concentrations at the last sampling period (24 h) were below 0.15μg/ml (lower quantifiable concentration) in 13 subjects. Figure 1 depicts the mean ± SD plasma concentration of rifampin for the 24 subjects. Rifampin dose was corrected to body weight to produce a dose range of 6.67 to 12.0 mg/kg (mean ± SD; 8.33 ± 1.23 mg/kg). The maximum rifampin plasma concentration (Cmax) was 8.86 ± 2.74 μg/ml andTmaxwas 1.88 ± 1.12 h. The elimination rate constant and the half-life of rifampin were 0.20 ± 0.05 h-1 and 3.70 ± 0.97 h, respectively (Table 1). The absorption rate was evaluated using the ratio Cmax/AUC0-∞. It is conventional to use Cmax as an index of absorption rate and the area under the plasma concentration-time curve as an index of extent of absorption. The ratio Cmax/AUC0-∞ has been proposed as an alternative index of rate because it corrects for extent and provides some information on absorption rate, but it is not sensitive to changes in the absorption rate constant [35]. This ratio is held to be a good parameter for the evaluation of absorption rates in comparative pharmacokinetic and bioavailability studies of immediate release drug formulations. This ratio ranged from 0.113 to 0.234 h–1(mean ± SD; 0.162 ± 0.035 h–1) for rifampin in this study. The MRT of rifampin averaged 7.25 ± 1.35 h (range 5.22–10.68 h). The oral clearance and volume of distribution at steady state of the drug averaged 0.162 ± 1.35 L/h.kg (range 0.089– 0.289 L/h.kg) and 1.21 ± 0.58 L/kg (range 0.54–3.08 L/kg), respectively.
| Population | No. of Subjects | Weight (kg) | Dose (mg) | Dose (mg/kg) | Ref. |
|---|---|---|---|---|---|
| Americans | 11 | 76.56±13.22 (53.2–99.1) |
600 | 8.07±1.48 (6.05–11.28) |
[38] |
| Mexicans | 8 | 65.6±7.0 (52.5–75.0) |
600 | 9.25±1.07 (8.0–11.43) |
[32] |
| Indians | 6 | NA | 600 | 10 | [24] |
| British | 10 | (54.0–89.0) | 600 | (6.7–11.1) | [26] |
| Italians | 10 | 67.6±3.86 (60.0–72.0) |
600 | 8.90±0.53 (8.33–10.0) |
[27] |
| Estonians | 19 | 23 19–29 |
600 | NA | [40] |
| Tunisians | 12 | 63.4±12.15 (48.0–88.0) |
300 | NA | [36] |
| Middle East | 24 | 73.4±9.9 (58.0–90.0) |
600 | 8.33±1.23 (6.67–10.34) |
This study |
NA: Not Available
Table 1: Pharmacokinetic studies of rifampin in different populations. Results are expressed as Mean ± SD and/or (range).
The mean plasma concentration of desacetylrifampin as a function of time for the 24 subjects following the administration of 600-mg rifampin capsules is shown in Figure 1. The pharmacokinetic parameters of this metabolite were also calculated for Middle Eastern subjects in this study. The maximum plasma concentration (Cmax, Met) of desacetylrifampin reached following the administration of 600-mg rifampin capsules averaged 0.96 ± 0.32 μg/ml, and the time to peak plasma concentration (Tmax, Met) averaged 4.29 ± 1.3 h. The elimination rate constant and the elimination half-life of desacetylrifampin were 0.1540.032 h–1and 4.68 ± 0.95, respectively. The area under the plasma concentration-time curves of desacetylrifampin up to the last measurable concentration (AUCMET,0-t) and to infinity (AUC MET,0-∞) averaged 8.738 ± 3.5 μg.h/ml and 9.998 ± 3.593 μg.h/ml, respectively. The mean value of the ratio between the area under the plasma concentration-time curves of desacetylrifampin and rifampin (AUCMET /AUCRIF)was 0.183 ± 0.052 (range, 0.109-0.339).
This study was conducted to determine the pharmacokinetic parameters of orally administered rifampin in Middle Eastern Arabs and compare them with those for other Middle Eastern Arabs living in North Africa. In addition, the results for Middle Eastern Arabs are compared with Mexican, Indian, Italian, British, Estonians and American Caucasian subjects. The results of the Tunisian study were normalized to 600 mg dose. The published plasma concentrationtime profiles for the 12 Tunisian subjectswere digitized to extract data points and analyzed assuming linear kinetics [36]. The elimination pharmacokinetics of rifampin can be safely considered linear in the dose range used in these studies and no report till now has described the kinetic profile of rifampin as nonlinear. The analysis produced almost identical figures for the pharmacokinetic parameters obtained for Middle Eastern Arabs living in Saudi Arabia. Figure 2 illustrates time courses of the mean plasma concentrations following the administration of 600 mg dose in Tunisian, Italian, Mexican, Estonians, British, American Caucasians and Middle Eastern Arab subjects [26,27,32,36,38,40]. Table 2 shows a comparison of pharmacokinetic parameters for all ethnic groups included. There were no statistically significant differences (p>0.05) in Cmax between Middle Eastern Arabs living in Saudi Arabia and North African Arabs living in Tunisia. Mann-Whitney “U” test indicated that the difference in Tmax for both groups is not statistically significant (p>0.05). The same conclusion was observed regarding other pharmacokinetic parameters except for t1/2 and Ket. The mean t1/2calculated for North African Arabs in Tunisia from the terminal portion of the digitized plasma concentration-time profiles was considerably longer (p<0.05) than that for Middle Eastern Arabs in Saudi Arabia. No statistically significant difference (p>0.05) in t1/2 between Tunisians and Italians, but t1/2 in both groups was statistically different (p<0.05) from other ethnic groups.
| Population | (h-1) | t1/2 (h) | Cmax(mg/ml) | Tmax(h) | AUC(0-t)(mg.h/ml) | AUC(0-∞) (mg.h/ml) | Cmax/AUC(0-∞) (h-1) |
|---|---|---|---|---|---|---|---|
| Americans (Caucasians) | 0.23±0.07 (0.0981-0.3742) |
3.35±1.37 (1.85-7.07) |
11.28±3.47 (6.2-17.36) |
2.09±1.58 (1.0-6.0) |
52.70±16.60 (27.4-96.3) |
56.81±16.34 (31.8-99.2) |
0.202±- 0.049 (0.123-0.288) |
| Mexicans | 0.25±0.08 0.18-0.38 |
2.98±0.82 1.82-3.96 |
13.51±5.02 4.30-18.04 |
1.88±0.83 1.0-3.5 |
NA | 73.61±28.66 18.31-111.72 |
0.189±0.031 0.160-0.235 |
| Indians | 0.21±0.144 0.09-0.48 |
4.67±2.54 1.45-7.83 |
11.84±2.61 8.3-14.4 |
2.0* | 52.98±12.52 34.63-65.63 |
76.88±29.04 36.71-115.40 |
0.163±0.048 0.125-0.22 |
| British | NA | 4.1* | 11.1*( 5.2-21.0) | 2.5* | NA | NA | NA |
| Italians | 0.371±0.105 0.09-0.46 |
2.34±1.92 1.50-7.80 |
11.59±3.31 7.3-17.5 |
2.30±0.72 1.5-4.0 |
76.73±21.03 51.9-108. | 77.6±21.15 51.9-108.9 |
0.152±0.029 0.102-0.19 |
| Estonians | 0.20±0.13 | 3.01±0.70 | 10.63±3.18 | 2.64±1.26 | 79.42±23.41 | NA | NA |
| Tunisians | 0.34±0.149 0.19-0.90 |
2.40±0.90 0.77-3.64 |
12.12±2.94 2.4-22.6 |
2.33±1.59 1.0-7.0 |
51.14±12.12 15.85-112.6 |
54.84±27.37 19.56-131.3 |
0.225±0.035 0.123-0.31 |
| Middle East | 0.20±0.05 0.12-0.30 |
3.70±0.97 2.34-5.63 |
8.86±2.74 3.47-14.23 |
1.88±1.12 1.0-6.0 |
53.89±16.62 27.07-78.58 |
55.77±16.96 29.71-81.93 |
0.162±0.035 0.113-0.234 |
*Median
NA = Not available
Table 2: Pharmacokinetic parameters of oral rifampin in normal healthy subjects from different populations. Results are expressed as Mean ± SEM and range.
The majority of clinical trials on drugs are usually conducted in the US and western European countries, therefore, the comparison of pharmacokinetics between Middle Eastern Arabs and Caucasians is of special interest. There were no statistically significant differences in clearance of rifampin between Middle Eastern Arabs and Caucasian Americans (p>0.05) (Table 3). On the other hand, the differences in the volume of distribution at steady state (Vss) between the two groups were highly significant (p<0.05).
| Middle Eastern Arabs | Caucasians | p (sig) | |
|---|---|---|---|
| CL (L/h) | 11.94±4.1 (7.49–21.36) |
11.28±3.07 (6.05–18.8) |
0.6065 (NS) |
| CL (L/h.kg) | 0.162±0.051 (0.089–0.289) |
0.15±0.038 (0.071–0.194) |
0.5502 (NS) |
| Vss (L) | 88.30±43.02 (41.27–228.03) |
54.28±25.42 (26.35–103.59) |
0.00497 (S) |
| Vss (L/kg) | 1.21±0.58 (0.54–3.08) |
0.735±0.425 (0.31–1.89) |
0.00998 (S) |
| MRT (h) | 7.25±1.35 (5.22–10.68) |
6.57±1.65 (4.13–9.07) |
0.2341 (NS) |
S: Significant; NS: Not Significant
Table 3: Comparison between Middle Eastern Arabs and Caucasians in pharmacokinetic parameters (mean ± SD).
Based on the first and second measurable concentrations (C1 and C2, respectively) on the plasma concentration-time curve, Peloquin et al reported that serum rifampin concentration models revealed that two groups of rifampin absorbers: smooth absorbers (C2 /C1 <4) and low absorbers (C2 /C1 <4) [23,24]. Using this definition, nine of the Tunisian subjects (75%) were smooth absorbers (median C2 /C1 ratio of 1.2), whereas 3 subjects were low absorbers (median C2 /C1 ratio of 5.3). On the other hand, 13 (54.2%) Middle Eastern Arab subjects were smooth absorbers (median C2 /C1 ratio of 2) and 11 (45.8%) subjects were low absorbers (median C2 /C1 ratio of 15.9). The majority of North American Caucasian subjects (81.8%) were smooth absorbers (median C2 /C1 ratio of 1.3). The median C2 /C1 ratio for Indian subjects was 1.39 (range 1.25-1.79) indicating that all 6 subjects were smooth absorbers.
Table 4 shows the frequency distribution of AUC values for all ethnic groups. It is evident that 83% of AUC values for Italian, Mexican and Indian subjects were between 50-110 μg.h/ml, and about 94% of AUC values for North American Caucasians, Tunisians and Middle Eastern Arabs were in the range of 20-80 μg.h/ml. One might therefore with justice argue that AUC spectrum for different populations can be divided into two distinct areas where the AUC of 60 μg.h/ml is probably the cutoff value. The examination of the frequency distribution of AUC vaues for all ethnic groups revealed a unique distribution of individuals within the specified intervals. Table 4 shows that American Caucasians, Tunisians and Middle Eastern Arabs can be grouped into a separate group of metabilizers, whereas Italians, Indians and Mexicans can be grouped into another distinct group. Analogous to the definition of Peloquin et al. of smooth and low absorbers of rifampin, the results of the present study suggest the existence of smooth absorbers (subjects with AUC < 60 μg.h/ml) and low absorbers (subjects with AUC > 60 μg.h/ml) [38,39]. Table 5 shows that, on the average, more than 70% of the Italians, Indians and Mexicans have AUC values greater than the proposed cutoff point of 60 μg.h/ml, whereas about 66% of the American Caucasians, Tunisians and Middle Eastern Arabs have AUC values less than the cutoff point. An attempt to apply the same principle toCmaxvalues for all ethnic groups revealed a less distict cutoff point (Table 5).
| Population | AUC (µg.h/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10-20 | 20-30 | 30-40 | 40-50 | 50-60 | 60-70 | 70-80 | 80-90 | 90-100 | 100-110 | 110-120 | 130-140 | |
| Italians | 3 | 2 | 2 | 1 | 2 | |||||||
| Indians | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Mexicans | 1 | 1 | 1 | 1 | 2 | 1 | 1 | |||||
| American | 1 | 1 | 7 | 1 | 1 | |||||||
| Tunisians | 1 | 2 | 3 | 3 | 2 | 1 | ||||||
| Middle East | 1 | 5 | 5 | 2 | 3 | 7 | 1 | |||||
| Total | 1 | 2 | 9 | 9 | 17 | 10 | 8 | 6 | 3 | 3 | 2 | 1 |
Table 4: Distribution of area under the curve in different populations.
| Population | AUC > 60 µg.h/ml | Cmax> 10 µg/ml |
|---|---|---|
| Italians | 7/10 (70.0%) | 6/10 (60%) |
| Indians | 4/6 (66.7%) | 3/6 (50%) |
| Mexicans | 6/8 (75.0%) | 6/8 (75%) |
| American | 2/11 (18.2%) | 6/11 (54.5%) |
| Tunisians | 3/12 (25.0%) | 8/12 (66.7%) |
| Middle East | 11/24 (41.7%) | 8/24 (33.3%) |
Table 5: Distribution of area under the curve in different populations.
These findings were supported by a subsequent analysis of the AUC distribution for all ethnic groups where the cutoff point was confirmed by means of a probit plot. The frequency histogram of the AUC (Figure 3) for all ethnic groups showed skewed unimodal but not bimodal distrbution indicating that AUC values were not normally distributed as assessed by Kolmogorov-Smirnov test (statistic=0.114, p=0.02375,). Probit transformation of the same data resulted in a clear bimodal probit plot with a breakpoint corresponding to an AUC of about 60 μg.h/ml. The probit plot of AUC showed two distinct lines with different slope. The first probit line corresponded to AUC range of 10-60 μg.h/ml and had a slope of 0.058 (r=0.997, p=0.00019), and the second probit line corresponded to AUC range of 70-140 μg.h/ml with a slope of 0.026 (r=0.992, p<0.0001). It is evident that the point at which the two probit lines crossed was taken as the cutoff value (60 μg.h/ml). These results clearly indicate the possibility of existence of polymorphism in rifampin pharmacokinetics. Plotting the probit of the combined frequencies of the AUC of American Caucasians, Tunisians and Middle Eastern Arabs produced a straight line parallel to the first segment of the line preceding the breakpoint for the combined probit line (corresponding to AUC 10-60 μg.h/ml). Similarly, the probit plot of the combined frequencies of AUC for Italians, Indians and Mexicans produced a straight line with no breakpoints parallel to the second segment of the combined probit line (corresponding to AUC 70-140 μg.h/ml). This analysis provides further supporting evidence of the intrinsic bimodality of the AUC data indicating the possible polymorphism in rifampin metabolism and pharmacokinetics.
Figure 3: Frequency distribution and probit plots of the AUC of rifampin in 71 subjects. The shaded areas represent AUC for 24 Middle Eastern Arab subjects (This study). The probit lines of the combined AUC for a) all subjects, b) American Caucasians, Tunisians and Middle Eastern Arabs, and c) Italians, Indians and Mexicans.
The analysis of desacetylrifampin data was only limited to Middle Eastern Arab subjects due to the lack of available data for other ethnic groups. There was a significant positive correlation between the AUC of rifampin and AUC of desacetylrifampin (r=0.695, p=0.00016), but there was no apparent trend for subjects with high AUC of rifampin to show a reduced AUC of desacetylrifampin over the entire Middle Eastern population. In consequence, calculation of the ratio ofAUC of desacetylrifampin to that of rifampin did not result in a separation of these individuals with higher AUC values of rifampin. Similarly, the calculation of the reciprocal ratio of the AUC of the metabolite to that of rifampin failed to reveal any distinct trend in rifampin metabolism among Middle Eastern Arabs.
In this report, an attempt was made to investigate the possible existence of interethnic differences in rifampin pharmacokinetics. Previous reports suggested the occurrence of such differences [31]. The biological basis for the observed higher values for AUC could be due to a genetically determined differences in metabolizing enzymes as a result of the difference in affinity or the decrease in the amount of enzyme expression. Also the increase in plasma protein binding cannot be ruled out. American Caucasians, Tunisians and Middle Eastern Arabs can be considered similar with respect to rifampin pharmacokinetics, while Italians, Indians and Mexicans have almost the same pharmacokinetic profile based on probit analysis of AUC values. The high increase in AUC in Indonesian subjects probably arose from the presence of dietary component such as spices which inhibit the metabolizing enzymes. It is well documented that the CYP superfamily consists of multiple individual members, with distinct characteristics, substrate activity and specific genetic regulation [41]. The expression of these enzymes can also be influenced by different environmental and life style factors which could lead to interindividual differences in expression and activity.
The lack of understanding of all the factors determining variability in pharmacokinetics of drugs and in response is perplexing to health care professionals as well as patients. Therefore, the process of prescribing medications such as rifampin, in fact, is an iterative process. Physicians usually start the patient with the so-called standard dose, then adjust the rifampin dose in response to the observed plasma level. Further investigations to using a large sample of subjects of various ethnic origins to unravel the probable effect of the underlying genetic determinants in pharmacokinetics of rifampin.
I wish to express my gratitude to Dr. Charles A. Peloquin, Director, Infectious Disease Pharmacokinetics Laboratory at the National Jewish Medical and Research Center, Denver, CO, for supplying the data for American Caucasians.