
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review - (2022)Volume 12, Issue 5
Our world is experiencing the passage through the post-COVID phase. Post-COVID syndrome known as “long COVID” refers to symptoms persisting for more than three weeks after the diagnosis of COVID-19 in a human subject. The main clinical presentations of COVID-19 include respiratory distress, shortness of breathing, muscle fatigability, fever, sneezing, loss of smell or taste, sore throat and pain associated with these conditions. A high portion of patients, who recovered this disease already, can manifest a plethora of long-lasting symptoms. Mostly, 25% of patients having COVID-19 may have characteristics extending beyond three weeks, and thus exhibiting the criteria of post-COVID syndrome. In this connection, primary healthcare providers can play a vital role in the management of patients with post-COVID syndrome. Although, many patients fully recover, health complications can delay a person’s complete return to a regular lifestyle. To resume normal life, COVID-19 survivors are required to take aid to routine consultations, physiotherapy, and dermatological care. Our focus of this review is not to limited only on the epidemiology, pathophysiology, and actual clinical characteristics for the various long-term syndromes that have been observed in each organ system in human body following the infection with SARS-CoV-2, but also to consider future directions, with regards to newer variants of this deadly virus and their potential impact on the longterm complications observed.
COVID-19; Post-COVID-19; SARS-CoV-2; Complications; Long-term; Prevention
The well-known Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) which is responsible for Coronavirus Disease 2019 (COVID-19) has spread across the world. Our universe has first seen the novel Coronavirus disease (nCoV-19) in human in Wuhan, China which was solely caused by the SARS-CoV-2 virus. Despite vaccination efforts and all necessary restrictions, SARS-CoV-2 has infected more than 1.2 million people in the United States alone, with a new wave of increasing cases partially due to novel variants, such as the Delta variant of the virus, which are more easily transmissible to human body [1-3]. Although the highest mortality rates had been seen primarily in the elderly population, as more of the vulnerable population became vaccinated, the spread of the virus shifted toward an unvaccinated, younger subject [4]. The most common clinical characteristics represented by human in their acute stage of this disease are fever, musculoskeletal symptoms (such as: fatigue, myalgia, and joint pain), dry cough, dyspnea, gastrointestinal symptoms, and anosmia with or without ageusia [5]. Most importantly, after viral infection different types of damage occur in multiple body organs, especially the brain [6]. In addition, peripheral and central inflammatory responses (neuroinflammation) may be also triggered by the infection, and can lead to long-lasting musculoskeletal problems, cognitive impairment, and psychological disorders such as: increased depression, anxiety, Post-Traumatic Stress Disorder (PTSD), and sleep problems [7-9]. Moreover, several potential life-threatening late complications also possible such as: lung fibrosis, Venous Thromboembolism (VTE), arterial thromboses, cardiac thrombosis and inflammation, stroke, “brain fog”, and dermatological complications [10]. More noticeably, an overall decline in quality of life has been observed even 1 year after major coronavirus attack [11]. Although, specifically in human the scope of presentations and studies of long-term complications is wide, but specific attributes of patients have been shown to be predictive of which symptoms they develop and for how long they sustain (Figure 1) [12].
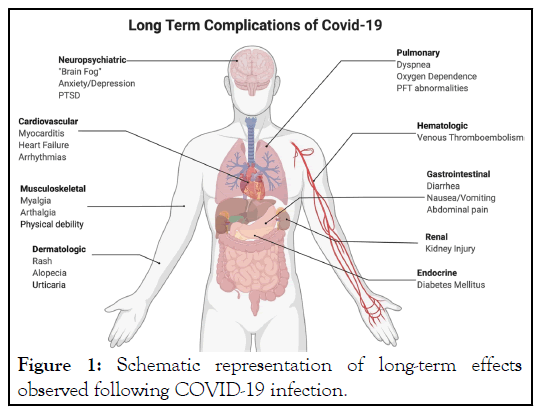
Figure 1: Schematic representation of long-term effects observed following COVID-19 infection.
Pathophysiology
Although SARS-CoV-2 primarily affects the lungs, it has been found to damage the vascular endothelium of several other vital organs of human. The explainable exact mechanisms responsible for long-term complications of deadly COVID-19 infection remain still unknown, but there are a number of strong proposed pathophysiological mechanisms of the virus that can be reasonable for these longer-term complications in human. So far, possible pathophysiological mechanisms may include direct viral tissue damage of host. Mainly, the entry receptor for SARS-CoV-2, Angiotensin-Converting Enzyme 2 (ACE2), is expressed in a variety of locations in the body allowing the virus to enter target cells through activation of its spike protein by transmembrane serine protease 2 [13,14]. These receptors are expressed in epithelial cells, nasal goblet cells, gastrointestinal epithelial cells, pancreatic beta cells, and renal podocytes suggesting that direct tissue damage may be a primary mechanism of the presentation of SARS-CoV-2 infection, which may also contribute to its longer-term complications [15-17]. Early studies revealed that, endothelial cells exhibited high expression level of “ACE2” and that ultimately led to substantial alteration to the integrity of the vessel barrier and promotion of a pro-coagulative state [18]. The long-term complications of these changes have been observed in follow-up studies of survivors of COVID-19, revealing pulmonary radiological abnormalities in 71% of patients and functional abnormalities in 25% of patients after 3 months period of COVID-19 infection [19]. Scientists also proposed several other clinical pathways leading to longterm COVID-19 infection complications. These include endothelial injury, immune system disruption, and hypercoagulability that often leading to fatal thrombosis. Immune system dysregulation has been suggested due to the finding of autoreactive T cells in autopsies of deceased individuals infected with COVID-19, likely due to mechanisms akin to those in autoimmune disease [20].
The respected authors of this review paper have searched articles carefully from various popular search engines like: SciFinder, Sci-Hub, PubMed, Web of Science, and Scopus databases. The selected articles on novel coronavirus have been collected in between from January 2020 to July 2022, by using the key words “coronavirus”, “SARS-CoV-2”, “novel coronavirus”, “COVID-19”, or “COVID-19” in combination with “long term”, “long COVID”, “Complications”, “Complexities” which were frequently modified as per the requirements for the search tool of each database. Articles were incorporated based on significance, priority, and originality with regards to the topics covered in this review.
In this case we will discuss mostly the prime post-COVID clinical complications by organ systems that affect the vital organs of infected human subjects.
Pulmonary
Various persistent and long post-COVID-19 respiratory symptoms are most common to the affected patients. We can see especially dyspnea, hypoxia. Decreased exercise capacity, running nose, sneezing, and cough, beyond 03 weeks from the onset of symptoms. Dyspnea is the most frequently respiratory reported symptom after COVID-19. Studies reporting respiratory symptoms from 1 to 12 months after COVID-19 show a prevalence of persistent dyspnea ranging from 5% to 81% after hospitalization [21-23] and about 14% in non-hospitalized patients with mild COVID-19 [24]. The persistence of dyspnea does not seem to be closely related to the initial severity of COVID-19. Indeed, dyspnea has been reported to be as frequent in patients who initially required initial Intensive Care Unit (ICU) admission. Clearly, dyspnea exerts a major effect on quality of life and socioeconomic status, as many patients with post-acute COVID-19 syndrome cannot return to their workplaces for 6 months after COVID-19. The mechanisms of dyspnea after COVID-19 are multifactorial, including parenchymal damage, dysfunctional breathing, cardiovascular dysfunction, and muscular deconditioning. Dyspnea progressively improves over time span of 01 year after COVID-19 attack.
Cough and sneezing seem to be less common than dyspnea, but they can persist for weeks or months after SARS-CoV-2 infection and has been reported in 2-42% of patients [25,26]. Due to the large number of patients with COVID-19 worldwide, the longterm respiratory complications of COVID-19 may lead to the major involvement and proper utilizations of health resources. Physicians and pharmacists should be aware of this condition and of the mechanisms that might lead to persistent respiratory problems in these patients to propose perfect individual management strategies adapted to each unique condition.
Hematologic
“Fatigue”, which is the sense of physical and mental weariness or exhaustion, slow response, lethargy, and inattention, one of the common symptoms reported to be experienced by patients by COVID-19 and continue to develop severe “post-COVID- 19 syndrome” [27]. Fatigue occurs to patients due to lack of oxygen carrying capacities of hemoglobin. VTE, a term referring to blood clots in the veins, is a serious but preventable hematologic condition that can cause disability and death to the COVID-19 patients. The etiology of this coagulopathy is multifactorial, including microvascular dysfunction and increased expression of tissue factors in response to inflammatory cytokines, as well as the effects of hypoxia on upregulation of hypoxia inducible transcription factors [28,29]. The exact long duration of hypercoagulability is unknown, but most VTE appears to occur within 2-4 weeks of infection [30-32].
Cardiovascular
Still long-term COVID-19 cardiac symptoms remain largely imprecise as well as inconsistent, which is not very surprising to us. Persistent symptoms may include palpitations, dyspnea, and chest pain. Palpitations and chest pain were reported in 9% and 5% of patients, respectively, evaluated at 06 months in a Chinese study [33], but they are of course not specific to any lesions. Left Ventricular Ejection Fraction (LVEF) <50% was detected at 4 months of the acute episode in 5% of nonintubated patients and 18% of patients who had received mechanical ventilation [34], but none had LVEF <40%. However, their previous heart condition was unknown. Interestingly, LVEF <50% was not associated with an increased incidence of persistent dyspnea. A recent publication from the French COVID cohort study group reported a similar prevalence of LVEF <50% and an impairment of diastolic function (8%) at 06 months following hospitalization for COVID-19 [35]. The incidence of cardiac anomalies varies, ranging from 60% to 30% of the patients studied at 3-4 months following the initial attack. These abnormalities may be present in patients who do not experience even acute cardiac manifestations [36-38]. However, the clinical consequences of these abnormalities are unknown.
In summary, while some patients certainly experience persistent cardiovascular abnormalities 3-6 months after the initial COVID-19-episode, large-scale studies that describe the exact incidence, consequences, risk factors and late evolution of these attacks are lacking.
Neuro-psychiatric
COVID-19 is responsible to lead many long-term neuropsychological disorders such as insomnia, anxiety, depression, stress, anger, sleep disturbances, fear, and Post-Traumatic Stress Disorder (PTSD) [39]. Fairly anxiety, depression, sleep disturbances and PTSD have been reported in 30-40% of COVID-19 survivors, like survivors of other pathogenic coronaviruses [40]. The pathophysiology of neuropsychiatric complications is mechanistically diverse and entails immune dysregulation, inflammation, microvascular thrombosis, iatrogenic effects of medications and psychosocial impacts of infection.
Finally, other interrelated factors, such as the severity of the initial infection, might account for some of the symptoms or sequelae. Indeed, profound hypoxemia and mechanical ventilation or extracorporeal membrane oxygenation procedures for patients admitted to the ICU might be associated with persistent cognitive dysfunction and psychological disturbances in the long term, which are perhaps associated with a risk of cerebral atrophy and ventricular enlargement. The role of the systemic manifestations and the management of long-term CNS consequences of COVID-19 remain to be investigated.
Renal
Renal involvement is frequent in long COVID-19, and the clinical presentation ranges from mild proteinuria to progressive acute kidney injury. SARS-CoV-2 might attack the kidneys directly, but the kidneys are also vulnerable to the uncontrolled inflammation and blood clots that are caused by this deadly virus. The International Society of Nephrology reported that kidney abnormalities are observed in 25-50% of patients with severe COVID-19 who require hospitalization. The number of patients who will go on to develop chronic kidney disease is currently unknown; however, a significant number might require dialysis or transplantation.
In one study, it was found that, about 35% of COVID-19 survivors had renal impairments at 6 months period, and 13% had new onset of renal dysfunction after having had normal kidney function during their initial illness [33]. Expected improved and better outcomes associated with close follow-up, those with kidney disease after acute COVID-19 should establish direct care with expert nephrologists [41,42].
Dermatologic
SARS-CoV-2 can be responsible for various long-term cutaneous manifestations via direct viral binding or secondarily through several allergic-immunologic mediated processes. Mostly every dermatological problem may be noticeable at phases of the prodromal, active, or convalescent of COVID-19 disease. Main dermatological signs specifically in adult subjects with COVID-19 include urticaria, maculopapular exanthem, papulovesicular exanthem, chilblain-like acral lesions, livedo reticularis or racemosa, and purpuric vasculitis [43]. Hair loss is the predominant symptom and has been reported in approximately 20% of COVID-19 survivors. Although the long- term dermatological problems can be easily amenable, but in most of the cases they are skipped and remain unnoticed by both patients and health care givers.
Gastro-intestinal and hepato-biliary
Surprisingly COVID-19 has the potential to modify the normal gut microbiome, including nourishment of opportunistic harmful organisms and depletion of beneficial groups. Common persisting gastro-intestinal symptoms of post-COVID patients include nausea, abdominal discomfort, diarrhea, vomiting, and loss of appetite which may persist up to one third of patients two months after discharge [33,44]. These persistent symptoms can be related to ongoing viral replication in the gastro-intestinal tract, resulting in the prolonged fecal shedding of SARS-CoV-2 seen even after respiratory samples become negative [45-48].
On the other hand, long-term unusual liver functions are frequently observed in post COVID-19 patients, which are assumed due to hepatocellular injury and/or biliary stasis [49,50]. Hepatic injury mainly occurs for the viral cytotoxicity, particularly in the biliary tree area and also for the adverse effects of anti-viral drugs consumption by patients [51]. In the case of COVID-19 survivors who previously had acute liver injuries, abnormalities in liver function can sustain but gradually improve over weeks to months [52].
Olfactory and taste
During the first COVID-19 wave, a substantial increase in olfactory and taste disorders (OTD: anosmia, hyposmia and ageusia) was observed in SARS-CoV-2-infected patients [53]. Thus, OTD was considered a prime diagnostic parameter for COVID-19 disease [54]. In patients with mild COVID-19, the estimated OTD prevalence ranges from 56.5% to 85.9%, according to the OTD evaluation method [55].
The mechanism that SARS-CoV-2 induces olfactory dysfunction may be related to the partial loss of olfactory receptor neurons in the olfactory epithelium and the cells which express two commonly known proteins used by SARS-CoV-2 to infect human cells (ACE2 and TPMRSS2). The mechanism by which SARS- CoV-2 leads to taste impairment is still unclear. It may be via direct damage of the gustatory organ, as ACE2 receptors have been identified in the mouth and on the tongue [56,57].
Endocrine
Based on the presence of COVID-19 virus in several endocrine glands [58], and expression of ACE2 in hypothalamus, pituitary, thyroid, gonads, and pancreatic islets in [59], researchers have come to conclusion that SARS-CoV-2 might affect the endocrine system in human.
The most prominent consequences of COVID-19 are abnormal glucose metabolism. Several arguments suggested the involvement of SARS-CoV-2 in the occurrence of abnormalities in glucose metabolism [60,61]. Onset of hyperglycemia, insulin resistance and β-cell hypersensitivity has been reported in one study of patients with COVID-19 without a history of diabetes [62]. In this study, among patients with new-onset hyperglycemia at hospital admission for COVID-19, about 35% of patients had persistent hyperglycemia in the next 06 months and diabetes was also diagnosed in 2% of patients.
Characteristics of human body are totally mysterious and at the same time the nature of COVID strains are very unique. Thus, they react in different patterns from patient to patient. It remains totally unclear whether these differences are due to discrepancies in treatment received or molecular mechanisms of viruses behind why certain individuals may be more predisposed to the development of long-term symptoms. Several important factors are yet to be addressed in the COVID-19. The broad impact of these novel strains on the development of long COVID-19 and ultimately which patients would be most affected, require further intense study.
Limitations of this review article include that we used most recently available published evidence from the hospitalized COVID-19 patients. But we believe a huge number of unrecorded non-hospitalized or un-diagnosed patients, could lead to a big discrepancy in our study. Moreover, information provided here for post-COVID syndrome is focused on current evidence, but it can be modified as more information would be available in the future.
No funding was received for this work.
There is no conflict of interest to declare.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hossain MM, Rahman MH, Tanmy TT, Kabir ML, Uddin KN (2022) Post-COVID Long-Term Complications in Human. J Clin Trials. 12:509.
Received: 26-Sep-2022, Manuscript No. JCTR-22-19357; Editor assigned: 28-Sep-2022, Pre QC No. JCTR-22-19357 (PQ); Reviewed: 12-Oct-2022, QC No. JCTR-22-19357; Revised: 19-Oct-2022, Manuscript No. JCTR-22-19357 (R); Accepted: 22-Oct-2022 Published: 26-Oct-2022 , DOI: 10.35248/2167-0870.22.12.509
Copyright: © 2022 Hossain MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.