Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Research Article - (2020)Volume 9, Issue 3
Variable quality of dosage forms of a wide range of drugs has often been reported, prompting the WHO to set standards for generic products. This study aimed to assess the quality and dissolution characteristics of generic ciprofloxacin 500 mg products available on the market, by comparison with a leading brand as well as to establish dissolution profiles in order to inform manufacturers and decision makers. A post-marketing quality assessment and comparative dissolution study of five generic ciprofloxacin products from different manufacturers available in Mongolia were completed. USP buffer at pH=1.2 (hydrochloric acid solution) and pH=4.5 (phosphate buffer solution) were dissolution media. In addition, weight variation, hardness, friability, disintegration time and assay were determined according to established methods. All five sampled products complied with the official specifications for uniformity of weight, friability and disintegration time. All five samples contained >99% (w/w) of labeled chemical content. However, significant inter and intra-brand variabilities in terms of dissolution rate were detected. As only two generic ciprofloxacin tablets included in this investigation were similar with the chosen comparator bioequivalence.
Quality control; ciprofloxacin hydrochloride; dissolution; interchangeability; Mongolia
API: Active Pharmaceutical Ingredient, BCS: Biopharmaceutical Classification System, BA: Bioavailability,BE: Bioequivalence, CDER: Centre for Drug Evaluation and Research, EMEA: European Agency for Evaluation of Medicinal Products, FDA: Food Drug Administration, MOH: Ministry of Health
The World Health Organization (WHO) has provided guidelines for global standardization and requirements for the registration, assessment, marketing, authorization and quality control of generic drug products [1,2]. Each country is responsible for the registration and marketing of generic products resulting in a wide range of requirements in individual countries [3].
Quality control requirements for tablet formulations include diameter, thickness, hardness, friability, weight variation, disintegration time and dissolution. The process of dissolutions evaluates the rate and level of drug release from its dosage form and becoming available for gastrointestinal absorption. Dissolution of a drug from its dosage from is dependent on many factors, which includes not only the physicochemical properties of the drug, but also the formulation of the dosage form and the process of manufacturing [4]. Laboratory testing of dosage forms available in the market is crucial to protect public health especially in low income countries [5], where counterfeit and substandard dosage forms are reported to be prevalent [6].
Ciprofloxacin, a synthetic fluoroquinolone derivative with a broad spectrum of antibacterial activity, is included in the Essential Drug List of Mongolia [7]. As of 2018, there were 43 tablet brands, 10 injectables, 11 ophthalmic preparations, manufactured by 17 different companies available in Mongolia [8]. According to the Biopharmaceutical Classification System (BCS), ciprofloxacin is currently classified as a Class IV drug, and drugs in this class are not suitable for the provision of “biowaiver” status.
Mongolia has been reported as one of the countries reporting high levels of antibiotic consumption [9], as well as substandard and falsified medicines being prevalent [10-12]. Currently, post marketing quality control and dissolution studies are not routinely performed. No studies thus far have assessed the dissolution profiles of generic medicines marketed in Mongolia. Therefore, this study aimed to assess the quality and dissolution characteristics of generic ciprofloxacin 500 mg products available on the market, by comparison with a leading brand as well as to establish dissolution profiles in order to inform manufacturers and decision makers.
Materials
Five commercially available leading brands of ciprofloxacin hydrochloride tablet 500 mg were conveniently purchased from the randomly selected retail pharmacies in Ulaanbaatar city in Mongolia.
A comparator product manufactured by Sandoz in Australia was kindly provided by Curtin University, Western Australia.
The samples were checked for their manufacturing license numbers, batch numbers, and manufacturing and expiry dates. The samples were blindly coded as Brand A, Brand B, Brand C, Brand D, Brand E and stored according to the stated conditions (Table 1). The labels of all the products claimed to contain 500 mg of the active ingredient per tablet as the hydrochloride.
| Trade name | Manufacturer | Manufactured date | Expiry date |
|---|---|---|---|
| Ciprofloxacin Sandoz 500 mg (comparator) | Australia | November, 2017 | November, 2022 |
| Ciprofloxacin 500 mg | Mongolia | February, 2018 | February, 2021 |
| Ciprofloxacin 500 mg | Russia | July, 2017 | July, 2020 |
| Ciprinol 500 mg | Slovenia | August, 2017 | August, 2021 |
| Cube 500 | China | April, 2016 | April, 2019 |
| Ciprolet 500 | India | August, 2017 | August, 2020 |
Table 1: Label information of selected brands of ciprofloxacin tablets
Uniformity of weight test
Uniformity of weight test involved weighing 20 tablets for each of the five brands individually using an electronic balance, then calculating the average weights and comparing the individual tablet weights to the average. The difference in the two weights was used to calculate weight variation by using the following formula:
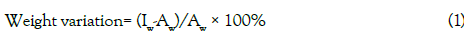
where, (Iw= individual weight of the tablet and Aw = Average weight of the tablet. The tablet complied with the test if not more than two individual weights deviated from the average weight by more than 5%.
Friability test
Ten tablets from each of the five brands were separately weighed and placed in a Roche friabilator and operated at 25 rpm for 4 min. The tablets were then dusted and weighed. According to the British Pharmacopoeia (BP) 2016 and the Mongolian National Pharmacopoeia (MNP) 2011, “A maximum loss of mass not greater than 1% was considered acceptable” [13,14].
Hardness test
Ten tablets were randomly selected from each of the five brands and tested. This test measures the pressure required to break when pressure is diametrically applied with a coiled spring.
Disintegration test
One tablet was placed in each of six tubes and the basket rack was positioned in a 1000 ml vessel containing 900 ml of water maintained at 37+0.5°C, so that the tablets remained 2 cm below the surface of the liquid on their upward movement and descended not less than 2 cm from the bottom of the beaker. The apparatus was operated for 30 min.
To comply with the BP and MNP standards, the tablets must disintegrate and all particles must pass through the 10 mesh screen within 15 min. Six tablets of each brand were tested.
Dissolution test
The dissolution test was undertaken using a tablet dissolution tester (Varian VK 7025). Six replicates for each brand were tested in USP buffer solutions at pH=1.2 (hydrochloric acid solution) and pH=4.5 (phosphate buffer solution). The medium was maintained at 37 ± 0.5°C. In all the experiments, 5 ml of dissolution medium was withdrawn at 0, 5, 10, 15, 30 and 45 min and replaced with an equal volume to maintain sink condition. Samples were filtered and assayed by a validated HPLC method (see below). The concentration of each sample was determined from a calibration curve obtained from an authentic sample of ciprofloxacin. As specified by the BP and MNP tablets meet with this test if not less than 80% is dissolved in 45 min [13,14].
Assay test
The assay test was prepared by crushing a total of 20 tablets of each brand in a motor and pestle. Powdered tablets equivalent to 2 g of ciprofloxacin were dissolved in 750 ml of the mobile phase, mixed with the aid of ultrasound for 20 minutes and diluted to produce
w w 1000 ml. A portion of the resulting suspension was filtered and the filtrate was diluted with sufficient mobile phase to produce a solution containing the equivalent of 0.05% w/v of ciprofloxacin. Ten μl solutions was injected in the HPLC and potency was calculated from the calibration curve constructed previously. Ciprofloxacin samples were analyzed by means of HPLC system (DGU-20A SHIMADZU, Japan) consisting of ODS Hypersil 150 × 4.6 mm 5 mkm, C18 μm column, UV 278 nm detector, flow rate: 1 ml/min, and phosphate buffer- acetonitrile/82:18/as the mobile phase.
Each brand of ciprofloxacin was determined according to BP and MNP. The BP and MNP specifications require the content of ciprofloxacin as hydrochloride should not be less than 95% and not more than 105% [13,14].
Drug release kinetics
To evaluate the kinetics of drug release from the tablets the results of the in vitro drug release study of formulations were fitted according to selected kinetic models including the zero- order, first- order, Higuchi and Hixson-Crowell models using the Kinet DS software [15]. The equations for the different release kinetics are given below:
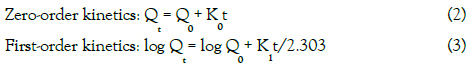
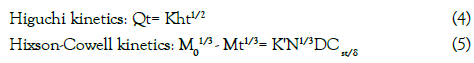
where, K0, K1and Kh indicates zero-order, first-order and Higuchi
rate constants respectively, Qt/Q0 is thefraction of drug released at time t, K is the rate constant and n the release exponent, N is number of particles, D is diffusion coefficient, Cs is the equilibrium solubility at the study temperature and δ is thickness of diffusion layer.
The kinetics that gives a high regression coefficient (R2) value was considered as the best fitted model [16].
To compare the dissolution profiles of the brands, a model independent approach evaluating the difference factor f1 and the similarity factor f2 were employed [17]. The difference factor f1 is the percentage difference between two curves at each point and is a measurement of the relative error between the two curves:
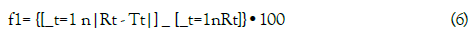
where n is the number of time points, Rt is the dissolution value of reference product at time t and Tt is the dissolution value for the test product at time t.
The similarity factor (f2) is a logarithmic reciprocal square root transformation of the sum of squared error and is a measurement of the similarity in the percent (%) dissolution between the two curves.
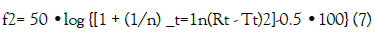
For two dissolution profiles to be considered similar, f1 should be between 0 and 15 while f2 should be between 50 and 100 [17].
Model independent estimations (difference factor (f1), similarity factor (f2)) were performed using the DD Solver program [18].
Data analysis
All results were expressed as mean ± SD. The results of the dissolution tests were analyzed by one-way analysis of variance (ANOVA) and Student’s t test. A p<0.05 was considered as significant.
The products showed notable differences when subjected to standard tests. The friability test showed all products lost>1% which is the limit considered acceptable. Brand B had the lowest mean friability and Brand D had highest mean friability [12]. The disintegration time for six brands of ciprofloxacin tablets were highly variable with the Sandoz product (comparator) not meeting the 15 minute standard. Detailed results of general quality control tests are shown in Table 2.
| Code | Average uniformity of weight (g) | Deviation from average weight (%) | Assay (%) | Hardness test (kg/cm2) | Friability (%) | Disintegration (min) |
|---|---|---|---|---|---|---|
| Sandoz | 543.2± 2.3 | 1.1±0.9 | 98.5± 0.1 | 0.5±0.4 | 16.8± 3.4 | 17.2±0.2 |
| A | 513.1±3.9 | 3.4± 0.2 | 96.8± 0.0 | 0.6±0.2 | 25.0± 4.8 | 2.8±1.1 |
| B | 507.8±4.1 | 0.8±2.1 | 100.3±0.1 | 0.4±0.1 | 32.1±3.3 | 8.8±0.9 |
| C | 511.2±3.3 | 2.5±0.8 | 101.1± 0.1 | 0.9±0.4 | 14.2±1.9 | 12.3±0.5 |
| D | 524.1±4.0 | 1.3±1.1 | 98.4± 0.1 | 0.9±0.2 | 12.2±2.6 | 13.8±1.3 |
| E | 501.1±1.8 | 1.8±0.8 | 98.1± 0.1 | 0.6±0.1 | 16.2±1.6 | 7.8±1.3 |
Table 2: Quality control test results of ciprofloxacin tablets
Dissolution profile
In vitro dissolution profiles indicated marked variations dependent on the dissolution media. In pH 1.2 HCl, the comparator product, no less than 85% of only Brand B and D were dissolved in 15 minutes whereas three remaining generic products were dissolved in 30 minutes.
Dissolution was slower in the phosphate buffer solution and 85% of Brand B and the comparator (Sandoz) were dissolved within 15 minutes. Other remaining products dissolved within 30 minutes (Figures 1 and 2).
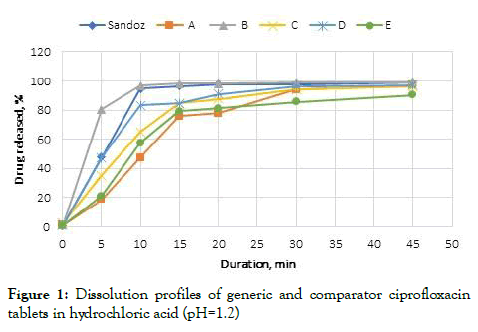
Figure 1: Dissolution profiles of generic and comparator ciprofloxacin tablets in hydrochloric acid (pH=1.2)
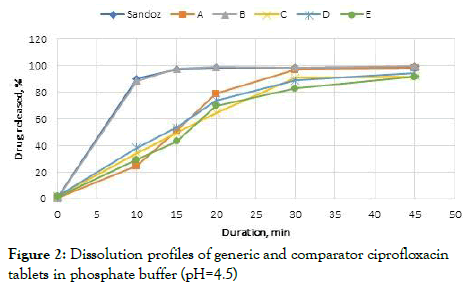
Figure 2: Dissolution profiles of generic and comparator ciprofloxacin tablets in phosphate buffer (pH=4.5)
Analysis of dissolution data
In order to investigate the release kinetics and mechanism, the dissolution data were fitted to the kinetic models including zero order, first order, Higuchi and Hixson models. In most cases in hydrochloric acid media, the first order model was the best fit for all brands (Table 3).
| Code | Zero order model | First order model | Higuchi | Hixson-Crowell | ||||
|---|---|---|---|---|---|---|---|---|
| HCl-0.1N | H3PO4 | HCl-0.1N | H3PO4 | HCl-0.1N | H3PO4 | HCl-0.1N | H3PO4 | |
| Sandoz | 0.92 | 0.93 | 0.96 | 0.93 | 0.92 | 0.93 | 0.87 | 0.88 |
| А | 0.8 | 0.64 | 0.9 | 0.64 | 0.8 | 0.64 | 0.95 | 0.62 |
| B | 0.93 | 0.85 | 0.93 | 0.84 | 0.93 | 0.85 | 0.88 | 0.92 |
| C | 0.57 | 0.93 | 0.86 | 0.93 | 0.57 | 0.93 | 0.74 | 0.77 |
| D | 0.83 | 0.85 | 0.98 | 0.98 | 0.83 | 0.85 | 0.94 | 0.95 |
| E | 0.91 | 0.91 | 0.98 | 0.97 | 0.91 | 0.91 | 0.96 | 0.96 |
Table 3: R2 values obtained from fitting the kinetic dissolution models of generic ciprofloxacin tablets marketed in Mongolia
Determining similarity and difference factors of ciprofloxacin tablets
Employing a model independent method, the similarity and difference factors were established for the drug dissolution profiles. The profiles were considered similar when f2=50-100 and f1=0-15 [19]. In the case of the hydrochloric acid medium, the tablets in the study were compared to the Sandoz. Dissolution profile; and Brand B (f2=59, f1=11) and C (f2=58, f1=15) were similar when compared with the comparator product manufactured by Sandoz in Australia (Table 4).
| Code | pH=1.2 | pH=4.5 | ||
|---|---|---|---|---|
| f2 | f1 | f2 | f1 | |
| Sandoz and A | 26 | 30 | 4 | 97 |
| Sandoz and B | 59 | 11 | 3 | 97 |
| Sandoz and C | 58 | 15 | 3 | 98 |
| Sandoz and D | 32 | 28 | 32 | 28 |
| Sandoz and E | 67 | 3 | 67 | 3 |
Table 4: Calculation of difference factor (f1) and similarity factor (f2)
The percentage dissolved was tested statistically to ascertain differences of dissolution release at 30 minutes among brands using one-way analysis of variance (ANOVA). The results of ANOVA indicated that the proportions dissolved were statistically significant different in pH=1.2 (Hydrochloric acid) and pH=4.5 (Phosphate buffer) (Table 5).
| Dissolution media | p- value |
|---|---|
| pH= 1.2 Hydrochloric acid | 0.010554* |
| pH = 4.5 Phosphate buffer | 0.009665* |
*p<0.05- statistically significant
Table 5: Statistical analysis of dissolution variations at 30 minutes
The present work has involved a post-marketing quality assessment and a comparative dissolution study of locally manufactured and imported ciprofloxacin tablets available in Mongolia. No branded version of ciprofloxacin was available in Mongolia at the time of study; however, Ciprofloxacin 500 mg manufactured by Sandoz in Australia was used as a comparator.
It is well-known that dissolution test is an established, reproducible, reliable and valuable tool for characterizing a drug product at different stages in its development and manufacture [20]. In addition, dissolution testing can be used to forecast the in vivo behavior of a drug, when the dissolution rate is rate-limiting as occurs with BCS I and III classes. In this study, general parameters of quality control were tested and all results were in compliance with BP and MNP requirements except for the disintegration results. However, a comparison of dissolution profiles using different approaches (model-dependent and model-independent) indicated that only 40% of commonly available ciprofloxacin tablets marketed in Mongolia were similar with those of a comparator product. The data for dissolution in hydrochloric acid would be an appropriate comparison, owing to the high solubility of ciprofloxacin at low pH values. Under these conditions it is expected that the dissolution rate would be the rate limiting process.
Comparative dissolution studies of ciprofloxacin tablets in different countries have been published. An Indian studyevaluated six generic ciprofloxacin tablets, manufactured by different companies and reported that all samples were bioequivalent with the chosen innovator brand [21]. Ngwuluka et al., evaluated six brands of ciprofloxacin 500 mg tablets available in Jos, Nigeria and found that only three brands (50%) may be used interchangeably with their chosen ‘innovator’ brand [22]. Similar to this study, a Lebanese study compared 10 brands of ciprofloxacin tablets and found significant variations among some brands in terms of hardness, disintegration and dissolution [23].
Several authorities including the United States Food and Drug Administration (FDA) and European Agency for the Evaluation of Medicinal Products (EMEA) have adopted the model-independent approach (similarity factor f2), as a criterion to compare the similarity of two or more dissolution profiles. Also, the BCS has included the estimation of a similarity factor (f2) as a guidance on Waiver of in vivo BA and BE Studies for Immediate-Release Solid Oral Dosage Forms [4]. The similarity factor (f2) is reported to be a simple and viable comparison approach to assess “bioequivalence” between two formulations [24]. In addition, the WHO recommends that if both strengths release 85% or more of the label amount of the Active Pharmaceutical Ingredient (API) in 15 minutes, using required dissolution media; the profile comparison with an model-independent test (similarity factor-f2, difference factor- f1) is unnecessary [19]. In this study, only two products (Brand B and
D) complied with these criteria, hence further comparison using similarity factor (f2) was applied.
The post-marketing quality control assessment, comparison of dissolution profiles and BE studies were not required in Mongolia at the time of this study, however the Drug Registration Regulation is currently being revised [25]. It is expected that the inclusion of BA and BE studies for locally produced and imported generic medicines prior to, as well as post marketing in Mongolia will become part of the quality assurance enforcement in Mongolia. In addition, the proposed approach is a cost-effective method that can be applied for both imported and locally manufactured products.
This study has shown significant variations occurred between commercially available ciprofloxacin tablets in Mongolia. Ciprofloxacin is a BCS IV drug and in vivo BE studies are required to confirm the interchangeability. Due to financial constraints and lack of resources BE studies are yet to be performed in Mongolia. However, in selected cases dissolution rates are recognized to be useful in developing an in vitro/in vivo correlation to reduce costs. Moreover, it is useful to establish the similarity of dissolution profiles pre and post-change products for SUPAC and allow approval of BE. This study was a pilot approach and further in vivo investigations are required in order to establish the interchangeability of generic products in Mongolia.
Not applicable
Not applicable
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
All authors declare that they have no competing interests.
No funding was received for the study.
SUPAC Scale-Up and Post-Approval Changes
WHO World Health Organization
Citation: Citation: Ganbat A, Dorj G, Sunderland B, Sanjjav T, Tompurev A, Luvsandorj T, et al. (2020) Post-Marketing Quality Assessment and Comparative Dissolution Study of Some Ciprofloxacin Tablets Marketed in Mongolia Drug Des 9:168.
Received: 10-Aug-2020 Accepted: 27-Aug-2020 Published: 03-Sep-2020 , DOI: 10.35248/2169-0138.20.9.168
Copyright: Copyright: © 2020 Ganbat A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.