
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2022)Volume 13, Issue 9
Background: Post-Operative Nausea and Vomiting (PONV) is an ongoing complication for operative care teams. PONV can result in patient distress (compromising their recovery profile) and discharge delays with associated cost implications. In 2019, the University Hospitals Birmingham NHS Foundation Trust had no published guidelines addressing PONV management. Aims were to determine the percentage of patients being risk assessed for PONV; discover the true incidence of PONV in this subset of patients versus predicted risk; to determine the current practices regarding prescription of preoperative, intra-operative and post-operative anti-emetics.
Methods: This was a prospective audit utilising intra-operative physical notes and online patient documentation. An initial review made it apparent that maxillofacial and Ear, Nose and Throat (ENT) operations present a higher risk of PONV. It was decided that surgical day-case patients in these specialities provided insight into current preventative and reactive anaesthetic practices. 71 patients were randomly identified as suitable to be incorporated into this study. For the final outcome, anaesthetists were asked to respond to a short online questionnaire.
Results: This audit found that no patients had been officially risk assessed for PONV as part of their pre-operative assessment. True incidence of PONV fell below the predicted risk of PONV based on each patient’s APFEL score. 80% of patients in this study were prescribed intravenous cyclizine post-operatively.
Conclusion: Following presentation of findings to anaesthetists and surgical colleagues at the Trust, we will propose the integration of the APFEL scoring system for PONV prediction into the preoperative anaesthetic assessment proforma.
Post-Operative Nausea; Antiemetic; Maxillofacial;
Post-Operative Nausea and Vomiting (PONV) is an ongoing complication which has significant implications for both patients and healthcare teams. PONV is defined as any nausea or vomiting experienced up to 24 hours after a surgical procedure [1]. In regards to patients, suffering PONV can result in distress thus compromising their recovery profile, and delays to their discharge with associated cost implications [1,2].
General incidence of PONV resides at 25% and so is a regular and substantive occurrence for healthcare teams in surgical aftercare [3]. PONV varies by patient population and is influenced by a variety of factors that are both modifiable and non-modifiable. Variables such as history of PONV or motion sickness, smoking status, length of surgery, intraoperative opioids and type of anaesthesia used can all impact on a patient’s actual risk [4].
Multiple methods have been developed to attempt to quantify each patient’s predicted risk by accounting for these factors, with the APFEL scoring system being most commonly adopted [4]. A score is calculated by attributing a point to specific recognized risk factors, with a higher score equivaling a more likely incidence of PONV.
However, despite PONV being a common complication, as of 2019 the University Hospitals Birmingham NHS Foundation Trust had no published guidelines addressing PONV management. Additionally, there is no existing pre-operative PONV risk assessment to aid in identifying those patients who would benefit from prophylactic antiemetic prescriptions. We defined prophylactic antiemetics to include both those given orally before surgery, and those administered intravenously during the operation.
The University Hospitals Birmingham NHS Foundation Trust has no published guidelines addressing PONV management. Therefore, this audit aims to examine the incidence of PONV relating to Ear, Nose and Throat (ENT) and Maxillofacial (MaxFax) patients at Queen Elizabeth Hospital. The Queen Elizabeth Hospital does not currently use a risk stratification system, so we will be applying the APFEL score to patients included in this audit [4].
Audit objectives include
1. To determine the percentage of patients being risk assessed for PONV.
2. To discover the actual incidence of PONV in ENT and MaxFax patients versus predicted risk.
3. To determine current practises regarding the prescription and administration of preoperative, intra-operative and post-operative anti-emetics.
A prospective audit was conducted at the Queen Elizabeth Hospital Birmingham (QEH) from 20th December 2018 to 19th January 2019. This audit was approved by the University of Birmingham and Queen Elizabeth Hospital Trust (QEH), who deemed the team access to patient, files prior to data collection. An initial review of potential study populations made it apparent that ENT and MaxFax operations present a higher risk of PONV [5]. Therefore, it was decided to collect patient data for adults undergoing day-case operations related to these specialities.
Inclusion criteria for this study were patients having ENT or MaxFax surgery at QEH from 20/12/2018 to 19/01/2019, and those procedures in ambulatory day-case. Exclusion criteria included patients aged less than 16 and untraceable patient notes due to early discharge.
Data collection occurred on 10 randomly selected days during specified month collection time. All patients who underwent an ENT of MaxFax day-case procedure on those days were incorporated. A final sample size of 71 patients from this population was identified with the aim of providing a snapshot view of current practice.
A data collection sheet was created to ensure all outcomes were addressed. The data collection sheets did not contain any patient identifiable information and full confidentiality was maintained throughout our data collection (Figure 1).

Figure 1: Data collection sheet.
Information was gathered from physical intraoperative patient notes and online patient profiles on ‘PICS’. Antiemetic administration data collection was gathered from the anaesthetic intraoperative booklet and the formal inpatient prescription chart. Additionally, we read nursing notes to ensure that the medications prescribed had been administered. A short knowledge survey was also sent out to anaesthetists to fully explore what is guiding current practice at QEH.
Demographics
During the data collection period, 71 patients were identified as suitable to be included in our study. Patient demographics such as gender, age, and smoking status were collected. Specifically, the age rage in this data set was 16-90 years, and in regards to sex 56% (N=40) were female.
The majority of patients recorded in this data set underwent ENT procedures (86%) compared to MaxFax surgeries. As shown in Figure 2, the distribution of MaxFax surgeries were more commonly dental (70%) over facial. Whilst there was more variety in the location of focus for ENT surgeries including ear, nose, throat and thyroid (Figure 2).
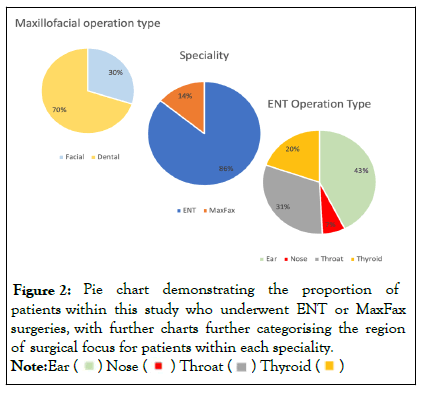
Figure 2: Pie chart demonstrating the proportion of patients within this study who underwent ENT or MaxFax surgeries, with further charts further categorising the region of surgical focus for patients within each speciality.
All MaxFax surgeries were under general anaesthetic, while 20% (N=12) of the ENT surgeries were under local anaesthetic as opposed to general anaesthetic.
Primary outcome-PONV risk assessment
University Hospitals Birmingham do not utilise predictive scoring for PONV, and so for this study we recorded the relevant patient demographics and calculated their APFEL score [4]. APFEL is scored on 4 recognized risk factors with each scoring one point-being female, a non-smoker, history of PONV/motion sickness and postoperative opioids.
Of our sample 40 were female, 45 were non-smokers, and 56 were prescribed opioids post-operatively. Each patient’s calculated APFEL score in this study is demonstrated (Figure 3).
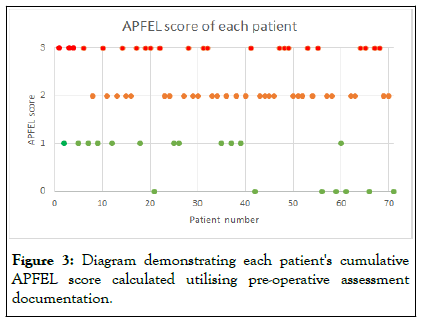
Figure 3: Diagram demonstrating each patient's cumulative APFEL score calculated utilising pre-operative assessment documentation.
However, our score estimation is limited to a maximum score of 3 as one of the recognizeds risk factors (PONV/motion sickness history) is not physically recorded in patient notes. We were unable to gather this information from the patients themselves due to the quick turn-around of postoperative recovery and subsequent discharge on the days of data collection.
Secondary outcome-true incidence of PONV vs. predictive risk
Each point on the APFEL score correlates with a relative increased predictive risk of PONV [4]. Those who score 0 are still predicted to have a 10% likely occurrence of PONV, with those scoring 3 (maximum possible with our patient cohort) having a 60% predictive risk.
Within this sample group, two patients experience one episode of vomiting postoperatively with APFEL scores of 1 and 2 respectively. Additionally, eight further patients reported postoperative nausea in recovery.
As demonstrated in Table 1, the true incidence of PONV for each APFEL score falls below the predicted incidence [4]. However, the true incidence of PONV follows the anticipated correlation that there is a high PONV incidence in patients with higher APFEL scores. Calculated Chi-square score of 1.77, with a P value of 0.62 and so the difference of true vs. predicted risk is not statistically significant (P<0.05) (Table 1).
| APFEL score | Predicted PONV risk (%) | Incidence PONV (number) | True PONV incidence (%) |
|---|---|---|---|
| 0 | 10% | 0/7 | 0% |
| 1 | 20% | 1/12 | 8% |
| 2 | 40% | 4/29 | 14% |
| 3 | 60% | 5/23 | 22% |
Table 1: Table comparing the predicted PONV risk versus the true PONV incidence in this study based on the APFEL score calculated for each patient.
Tertiary outcome-antiemetic administration practices
In this study we gathered information on antiemetics prescribed preoperatively, intraoperatively, and postoperatively. Those given before and during the surgery we defined as prophylactic antiemetics. On prior reading, it was documented that ondansetron (5-HT3 antiemetic) is a common prophylactic antiemetic [1], however in our sample set a variety of drugs were administered prophylactically.
The drugs administered prophylactically included intravenous cyclizine, ondansetron and dexamethasone. All patients received these drugs intravenously at the same doses (cyclizine 50 mg, ondansetron 4 mg, dexamethasone 6.6 mg), apart from one patient who was documented to have received 8 mg of both ondansetron and dexamethasone.
The majority of patients (39.4%) received both ondansetron and dexamethasone as prophylaxis. A significant proportion of this sample set (22.5%, N=16) received no antiemetics preoperatively or during the surgery. All antiemetic prophylaxis was administered intraoperatively and intravenously, no antiemetics were provided to the patients of this sample set preoperatively (Figure 4).
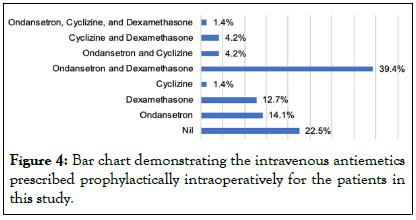
Figure 4: Bar chart demonstrating the intravenous antiemetics prescribed prophylactically intraoperatively for the patients in this study.
Of those patients who experienced PONV, 80% of these patients were prescribed 50 mg of intravenous cyclizine. One patient was documented to have been given a sick bowl before nausea spontaneously resolved. Cyclizine was the only antiemetic provided post-operatively for PONV treatment.
The sample size of this audit was limited by the short collection period of 10 randomly selected over one month. As a snapshot study for an initial insight into PONV practises, the study was sized appropriately. However, in order to make conclusions for an entire surgical population a larger study will be required across specialities with thus a larger sample size. Additionally, the patients selected to be incorporated were from surgical subspecialties deemed to be at higher risk of PONV, however there are other groups of patients who have been found to have a higher incidence of PONV. For example gynaecological patients who due to the ‘head-down’ position intraoperatively have been reported to have increased PONV incidence [5,6]. If this study were to be repeated, widening the patient cohort to other surgical specialities may be more beneficial when concluding on a wider subset of patients.
In regards to the primary aim, we found that none of the patients were risk assessed for PONV using a stratified risk scoring system prior to their procedure. Healthcare professionals carried out the standardised preoperative assessment booklet for new surgical admissions which included collecting data regarding relevant risk factors to PONV, which could have had the potential to guide antiemetic prohopylaxis judgement. However, in the absence of a qualitative score and departmental algorithm for management of those at higher risk, it is unclear how this impacted upon practice. In particular, our data found no correlation between our applied APFEL scores and prophylactic measures used on individual patients.
Secondly, the actual incidence of PONV observed was 14% which falls below the reported incidence of 30% without prophylactic measures used [7-9]. There was some correlation between patents’ predicted risk based on APFEL scores and the incidence of PONV. Of the patients who experienced PONV, 90% had an APFEL score of 2 or more, indicating the high risk of PONV (>40% incidence risk). The limitation of our calculated APFEL score has been previously alluded to whereby we were unable to record on the fourth risk factor-PONV/motion sickness history. This has resulted in a slew of results, so patients may have had a higher predicted risk than anticipated. Also, those scoring in each quartile may in fact belong in a higher predictive risk category.
Furthermore, PONV is defined as up to 24 hours postoperatively. However, all patients were deemed fit for discharge in this study by healthcare professionals if they were nausea free for one hour. This was recorded in each patient’s discharge report on ‘PICS’. Therefore there is insufficient follow up for these patients as PONV could still have occurred following discharge. Therefore, in future studies day-case surgical patients may not be an appropriate group to study as they cannot be truly recorded as nausea free for 24 hours.
In regards to the third outcome, we ensured we collected full information on all drugs administered. This included the drug administered, its dose, time given, and the route of administration. As to what guided this practice we sought further information from the anaesthetists themselves and did this via an anonymous feedback form. Therefore, when creating a future guideline for antiemetic prophylaxis all information in this case has been independently gathered and verified by nursing notes for administration.
Questionnaire
Following data collection, an anonymous questionnaire was sent out to the anaesthetists at QEH. The purpose behind this was to gain insight into what is guiding the antiemetic practises. It asked which antiemetics they utilised prophylactically and when they administer them, whether they risk assessed people for PONV and how and whether they were aware of any PONV guidance. Overall we received 34 fully completed responses within 5 days of sending out the survey.
The first question addressed what guided their practice, and it was discovered that the majority of anaesthetists (85.29%) were guided by their experience and training. Other feedback included that NICE guidance and the APFEL score itself governed their PONV related practice.
In this survey it was recorded that 50% of anaesthetists agreed that patients were risk assessed for PONV prior to surgery, however we found no documentation of the aforementioned risk assessment in patient notes. Additionally, 81.8% of these anaesthetists said they never prescribed antiemetics preoperatively, with the remainder prescribing ondansetron or dexamethasone prior to surgery if it was deemed necessary.
All bar 1 one the anaesthetists stated that they always prescribe antiemetics intraoperatively, but the specific drug provided varied within this data set. Over 95% of this group initially prescribed ondansetron and dexamethasone, with fewer numbers choosing to provide cyclizine, droperidol, and prochlorperazine in descending order. Their reported medications of choice matched what our patients were prescribed.
All of the responses stated that PRN antiemetics were prescribed post-operatively, with over 85% of these prescriptions being for cyclizine and ondansetron. In addition, prochlorperazine and dexamethasone were also prescribed for this function.
We discovered when researching the supporting evidence for PONV treatment guidance that the trust of University Hospitals Birmingham do not have one addressing this matter. Hence it was a surprise that 30% of our group reported that they were aware of a guideline specific to this trust. Therefore it is important to emphasise when presenting this study that there is a lack of an official guide for antiemetic prescription in relation to surgical procedures overall.
The development of a trust guideline for the assessment and management of PONV would provide a framework for standards comparison and re-audit in the future. We suggest that the APFEL scoring system could be incorporated into the existing preoperative admission notes. Potentially, the score could be calculated automatically in ‘PICS’ using details already collected such as their smoking status, sex, and planned postoperative opioids. Then, utilizing relevant systematic reviews in agreement with current anaesthetic practice, an official antiemetic regimen for PONV prevention could be trialled within QEHB. Following this, actual benefits of PONV prediction and prevention can be concluded.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Binnie C, Dicks-Ilori L, Hasan K (2022) Post-Operative Nausea and Vomiting (PONV) Management in Adult ENT and Maxillofacial Patients. J Anesth Clin Res. 13:1084.
Received: 03-Oct-2022, Manuscript No. JACR-22-19787 ; Editor assigned: 05-Oct-2022, Pre QC No. JACR-22-19787 (PQ); Reviewed: 19-Oct-2022, QC No. JACR-22-19787 ; Revised: 26-Oct-2022, Manuscript No. JACR-22-19787 (R); Accepted: 04-Nov-2022 Published: 04-Nov-2022 , DOI: 10.35248/2155-6148.22.13.1084
Copyright: © 2022 Binnie C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.