Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2021)
Purpose of Review: Hepatitis B virus infection and the immune response in babies and adults.
Findings: The presence of myeloid-derived suppressor cells (MDSCs) in new-borns makes Hepatitis B to become chronic over years with both antigens present in the blood, HBs and HBe, the immune system not being able to recognize the infection in the first years of life. The expansion of myeloid-derived suppressor cells (MDSCs) allows the immune system to maintain the infection with Hepatitis B, although the patient reaches adult age, because myeloidderived suppressor cells (MDSCs) are responsible for the disfunction of the immune system and not the virus itself. Our goal is to show what is maintaining T cell exhaustion in hepatitis B, why this is happening and what is responsible for this.
MDSC is responsible for chronic Hepatitis B infection. The expansion of MDSC is the format in which hepatitis B manage to escape the immune system’s response. MDSC cells have the ability to interact with these signals generated by common progenitor lymphoid cells and in this way the immune system cannot exercise its function.
Hepatitis B; MDSC; Immune system; Antigen HBe; Lymph node; Inhibitory
Hepatitis B is the most common serious liver infection in the world. It is caused by the hepatitis B virus that attacks and injures the liver.
Two billion people (or 1 in 3) have been infected and about 290 million people are living with a chronic hepatitis B infection.
Each year up to 1 million people die from hepatitis B despite the fact that it is preventable and treatable.
The human hepatitis B virus belongs to the family of hepadnaviridae. The hepadnaviridae are subdivided into mammalian and avian hepadnaviruses. The mammalian hepadnaviruses include Human Hepatitis B Virus (HBV), Woodchuck Hepatitis Virus (WHV) and the ground squirrel hepatitis B virus (GSHV). The Duck Hepatitis B Virus (DHBV) and the Heron Hepatitis B Virus (HHBV) belong to the avian hepadnaviruses [1].
Structure of the Hepatitis B Virus
HBV has a small (ca. 3.2 kbp), partially Double-Stranded (DS), Relaxed Circular (RC) DNA genome [2] which is replicated via reverse transcription from an RNA intermediate, the pregenomic RNA (pgRNA) [3]. The RC DNA genome is packaged into an icosahedral capsid composed of the HBV core protein (HBc), which in turn is enclosed in an envelope layer composed of host cell derived lipid bilayer studded with three viral envelope or surface proteins [4] or "HBcAg," which contains the hepatitis B virus DNA and enzymes used in viral replication.
Life cycle of the Hepatitis B Virus
The hepatitis B virus (HBV) has a complex life cycle. The virus enters the host liver cell and is transported into the nucleus of the liver cell. Once inside the nucleus, the viral DNA is transformed into a covalently closed circular DNA (cccDNA), which serves as a template for viral replication (creation of new hepatitis B virus). New HBV virus is packaged and leaves the liver cell, with the stable viral cccDNA remaining in the nucleus where it can integrate into the DNA of the host liver cell, as well as continue to create new hepatitis B virus. Although the life cycle is not completely understood, parts of this replicative process are error prone, which accounts for different genotypes or “genetic codes” of the hepatitis B virus.
A hepatitis B infection can result in either an acute infection or a chronic infection. When a person is first infected with the hepatitis B virus, it is called an "acute infection" (or a new infection).
The risk of developing a chronic Hepatitis B infection is directly related to the age at which a person is first exposed to the Hepatitis B virus. The younger a person is when they are first infected, the greater the risk of developing a chronic Hepatitis B infection (Figures 1-10).
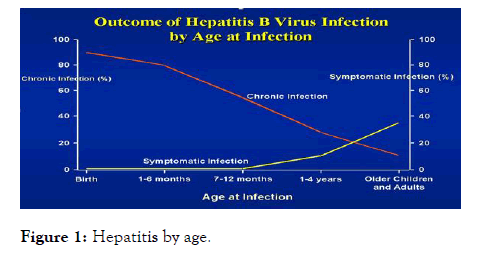
Figure 1: Hepatitis by age.
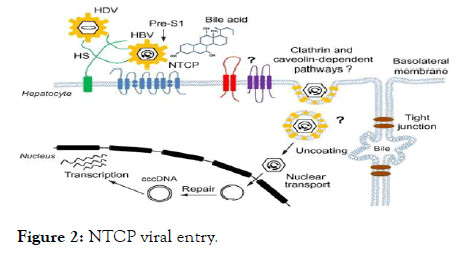
Figure 2: NTCP viral entry.
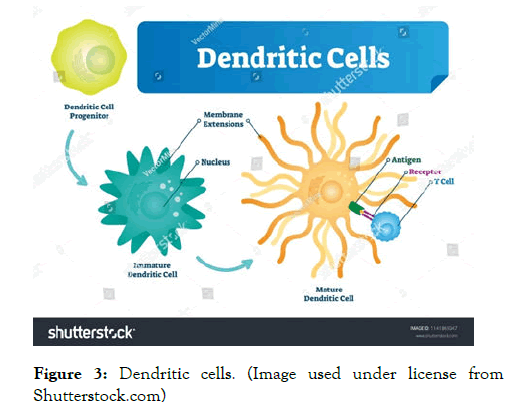
Figure 3: Dendritic cells. (Image used under license from Shutterstock.com)
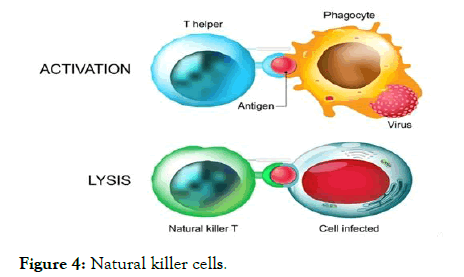
Figure 4: Natural killer cells.
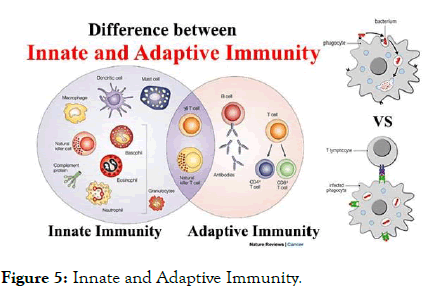
Figure 5: Innate and Adaptive Immunity.
Figure 6: Myeloid derived suppressor cells. (Image used under license from Shutterstock.com)
Figure 7: MDSCs functions. (Image used under license from Shutterstock.com)
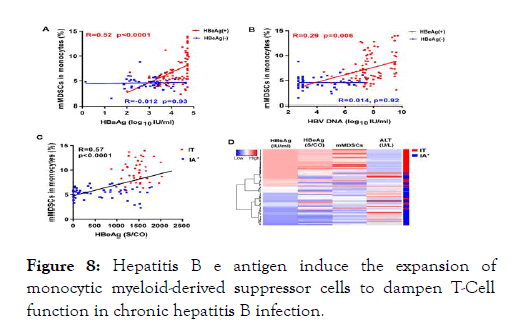
Figure 8: Hepatitis B e antigen induce the expansion of monocytic myeloid-derived suppressor cells to dampen T-Cell function in chronic hepatitis B infection.

Figure 9: Myeloid derived supresor cells, immunosuppressive function and tolerance.
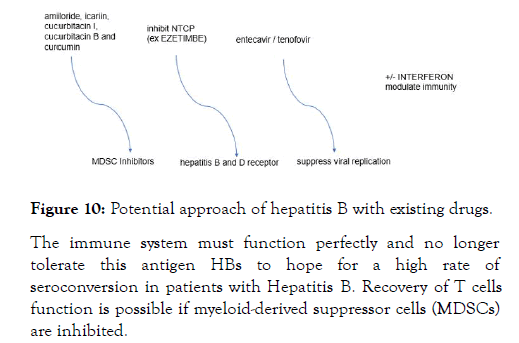
Figure 10: Potential approach of hepatitis B with existing drugs.
Antiviral therapy
The goals of antiviral treatment are to decrease the morbidity and mortality related to CHB. The achievement of a sustained suppression of HBV replication has been associated with normalization of serum ALT, loss of HBeAg with or without detection of (anti-HBe), and improvement in liver histology. Historically, the term “cure” was avoided in treatment of CHB, given that persistence of covalently closed circular DNA (cccDNA), the transcriptional template of HBV [5,6], in the nucleus of hepatocytes, even in persons with serological markers of resolved infection, poses a lifelong risk for reactivation of infection. However, an immunological cure may be defined by HBsAg loss and sustained HBV DNA suppression and a virological cure defined by eradication of virus, including the cccDNA form. The latter is not currently an attainable goal. There are six therapeutic agents approved for the treatment of adults with CHB in the United States and five therapeutic agents approved for the treatment of children with CHB. Side effects are more frequent with interferon (IFN) therapy than with nucleos(t)ide analogs (NAs) therapy. Overall, all NAs have an excellent safety profile across a wide spectrum of persons with CHB, including those with decompensated cirrhosis and transplant recipients [7].
For persons with HDV coinfection, the only effective treatment is pegylated interferon (Peg-IFN). For persons with HIV coinfection, treatment of HBV needs to be coordinated with HIV therapy given that several HBV drugs have anti-HIV activity (tenofovir, entecavir, lamivudine, and telbivudine). Biochemical, serological, virological, and histological endpoints are used to assess the success of therapy. Assessments are performed on continuous therapy [8,9] and after therapy discontinuation (PegIFN) [10-12]. The best predictor of sustained remission offtreatment is HBsAg loss, but this is infrequently achieved with current therapies.
Sodium taurocholate co-transporting polypeptide (NTCP) has been identified as a functional receptor for hepatitis B virus (HBV).
HBV enters host hepatocytes through a multi-step process that is initiated by low-affinity viral attachment to host hepatocytes [13]; this attachment is followed by specific and high-affinity interaction of the HBV large surface protein (LHBs) with an entry receptor, sodium taurocholate co-transporting polypeptide (NTCP), via the preS1 region of LHBs. The liver bile acids transporter sodium taurocholate co-transporting polypeptide (NTCP) is responsible for the majority of sodium-dependent bile salts uptake by hepatocytes. NTCP also functions as a cellular receptor for viral entry of Hepatitis B virus (HBV) and Hepatitis D virus (HDV) through a specific interaction between NTCP and the pre-S1 domain of HBV large envelope protein.
The finding that NTCP functions as an HBV entry receptor has enabled discovery efforts to identify agents that specifically inhibit HBV entry. To date, Myrcludex-B, CsA, irbesartan, ezetimibe, ritonavir, and vanitaracin A have been reported to inhibit HBV infection by directly targeting [14-32]. However, all of these agents have the potential to inhibit the function of NTCP for uptake of bile acids into hepatocytes.
A large number of clinical studies have shown that chronic HBV persistent infection causes the dysfunction of innate and adaptive immune response involving monocytes/macrophages, dendritic cells, natural killer (NK) cells, T cells.
Dendritic cells (DCs) in Hepatitis B
Dendritic cells (DCs) are the most important antigen-presenting cells, and are divided into myeloid dendritic cells (mDC) and plasmacytoid dendritic cell (pDC).
DCs bridge the innate and adaptive immunities, and consequently play pivotal roles in eliminating external antigens.
Dendritic cells capture antigens and deliver to lymph node.
Dendritic cells initiate inflammation.
During HBV infection, mDCs and pDCs display functional defects, which influence the cross talk with NK cells, making inefficient the NK cells activation.
DCs also have an important role in the induction and maintenance of tolerance.
The difference between immature and mature DCs is distinctly based on variations occurring on a phenotypic level and functional level.
Dendritic cells (DCs) are the professional antigen presenting cells, which process and present antigen to T cells, and are involved in the production of cytokines that influence T-cell polarization.
The studies of DCs subsets in chronic HBV infection have primarily been limited to myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), two populations isolated from the peripheral blood. The frequency of mDCs in CHB patients’ shows a reduction which could be recovered by antiviral therapy.
Natural killer (NK) cells are essential in the early immune response against viral infections, especially by eliminating virus-infected cells.
Instead, viruses have evolved multiple mechanisms to escape NK cell-mediated viral clearance.
Because new-borns rely heavily on the innate immune system, we evaluated the cellular phenotype and function of some of the first cellular responders during infection, the natural killer (NK) cells.
Due to limited antigen exposure in utero, new-borns rely heavily on the innate protection system.
Natural killer cells (NKs), a component of this system, are among the first cellular responders to viral infection.
So, NK cells in the heart blood are less effective than adult NK cells in initiating cell death in their targets.
Umbilical cord blood, a convenient source of cells representative of the new-born’s circulation, has facilitated the comparison of new born and adult NK cells.
Human NK cells are generally defined as a group of lymphocytes that express CD56 but lack the T cell receptor (TCR) - CD3 complex. They represent about 15% of all peripheral blood lymphocytes and this proportion can rise to more than 30% in the liver [33]. NK cells exert their effects mainly through the recognition and killing of target cells and the secretion of cytokines such as interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), which can modulate antiviral immune responses. By producing antiviral cytokines and chemokines, NK cells also play an important role in bridging the innate and adaptive immune responses. Since the immune system is a reticular and syntrophic entirety, NK cell dysfunction also influences the function of the other parts of the system, especially effector T cells. CD8 T cells are the main effector cells responsible for viral clearance and disease pathogenesis during acute HBV infection.
Other players of the innate immune system, natural killer (NK) cells, are activated early during infection, before HBV-specific T cells arise [34]. Later on during infection, functionally active HBV-specific T cells can be detected, which are thought to play an important role in viral clearance. In chimpanzees, depletion of CD8+ T cells at week 6 of infection leads to failure to clear the infection [35]. During chronic infection, HBV-specific T cells are exhausted and their function is impaired.
Monocytes/macrophages are important natural immune cells found in peripheral blood and organ tissue, and play multiple roles in the innate and acquired immune responses [36]. Monocytes/macrophage interact with lymphocytes through inhibitory or activating surface molecules. HBV stimulates monocyte/macrophage secretion of transforming growth factor β (TGF-β) and interleukin-10 (IL-10), while inhibiting the secretion of tumour necrosis factor α (TNF-α) and IL-12 induced by toll-like receptor (TLR2).
Myeloid-derived suppressor cells induce regulatory T cells in chronically HBV infected patients.
Myeloid-derived suppressor cells (MDSC) are pathologically activated and relatively immature myeloid cells, which are implicated in the immune regulation of many pathologic conditions [37,38]. Phenotypically and morphologically MDSC are similar to neutrophils (PMN-MDSC) and monocytes (MMDSC). However, they have potent suppressive activity, a distinct gene expression profile, and biochemical characteristics [39].
Recent studies have found that MDSCs play a critical role in chronic HBV infection. The level of peripheral MDSCs in chronic Hepatitis B (CHB) patients was significantly higher than that of healthy adults, and the percentage of MDSC cells had a significant correlation with HBV load in the plasma of HBV patients [40] and the mouse model.
The immunosuppressive functions of MDSCs are directed mainly at T cells; however, reports suggest that they also regulate B cell immune responses, DC-mediated immune responses, and macrophage-mediated immune responses [41-43].
In addition, MDSCs produce suppressive cytokines IL-10 to inhibit T-cell response in CHB patients [44].
MDSC not only directly inhibits T cell response through such mechanisms as arginase but also indirectly influences immunomodulatory function by inducing regulatory T cells (Treg) [45,46].
However, in pathological situations, such as chronic infection or cancer, MDSCs induce a profound immunosuppression and may therefore support tumour progression.
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population of immature myeloid cells with broadly distinct phenotypes that fail to terminally differentiate into granulocytes, macrophages, or DCs.
They exhibit a remarkable capacity to inhibit immune responses mediated by T, B, and NK cells.
In chronic HBV infection, the antiviral functionality of NK cells is also impaired, evidenced by an alteration of the phenotype and the receptors of NK cells.
This inhibition of NK cell activity is mainly mediated by myeloid-derived suppressor cells (MDSCs) via NKp30 receptor on NK cells and pro-inflammatory cytokines.
In addition, accumulated liver MDSCs due to HBV infection suppress CD8+ T cell function and promote systemic CD8+ T cell exhaustion, characterized by high expression levels of inhibitory receptors such as CTLA-4, PD-1, and TIM-3 [47].
However, they usually express the common myeloid markers CD33 and CD11b but lack expression of markers for mature myeloid cells, such as HLA-DR [48,49].
Due to the heterogeneous nature of these cells, MDSCs can be further divided into 2 major subsets: monocytic (M-MDSC) and granulocytic (G-MDSC). For human MDSCs, the monocytic subset contains CD14+ cells, while the granulocytic subset contains CD14− but CD15+ cells.
Furthermore, they inhibit CD4+ T cells and metabolically regulate HBV-related liver damage [50] MDSCs can induce the development of immunosuppressive regulatory T cells (Tregs) during chronic HBV infection primarily via a TGF b and the IL-10-dependent signalling pathway [51].
Tregs specifically inhibit CD8+ T cell activity; further blocking HBV-specific immune responses, leading to HBV persistence. On the other hand, low levels of HBV activity controlled by HBV antigen-specific CD8+ T cells lead to sustained liver inflammation and the functional depletion of HBV antigenspecific CD8+ T cells [52,53].
While a tolerant, anti-inflammatory state is likely advantageous for full-term viviparity [54] its persistence after birth may contribute to the reduced ability of infants to respond to infections and vaccinations in early life. However, they have potent suppressive activity, a distinct gene expression profile, and biochemical characteristics [55]. None or very few MDSC are observed in steady state physiological conditions.
MDSCs can directly or indirectly (by interacting with several components of both innate and adaptive immunity) contribute to the induction of an immune suppressive environment [56,57], and angiogenesis [58-60].
HBeAg is not a structural component of HBV and is not essential for viral DNA replication, however, HBeAg positivity is associated with high levels of viremia in patients. HBeAg may represent a viral strategy to establish persistent infection, but the mechanism remains largely ambiguous. Growing evidence suggests that chronic HBV infection may be shaped by MDSCsmediated T-cell exhaustion.
To assess whether HBeAg- induced CD33+ MDSCs impairs T-cell functions, we incubated PBMCs from healthy donors with or without rHBeAg (control) for 5 days. CD33+ cells were then isolated, and HLA-DR, CD11b and CD14 were analysed by flow cytometry. A significant decrease of surface expression of HLADR, low levels of CD11b, and equivalent levels of CD14 was observed on HBeAg-induced CD33 cells compared to control CD33 cells. As shown in Fig 5A and 5B, HBeAg-induced CD33+ MDSCs markedly decreased CD8+ and CD4+ T cell proliferation compared to control CD33+ cells, indicating an inhibitory effect of HBeAg- induced MDSCs on T cells [61,62].
As we have seen in the above articles, the Hepatitis B virus causes a dysfunction in the entire immune system.
The purpose of this paper is to shown what happens in the immune system of babies and adults in hepatitis b infection. We believe that research in the right direction will help pharmaceutical companies to accelerate the development of optimal solutions. Our goal is to show what maintains T cell exhaustion in hepatitis B, why this is happening and how is happening.
The purpose of this paper is to show that in fact the immune system itself through the presence and expansion of myeloidderived suppressor cells (MDSCs) maintains the hepatitis infection, and the MDSC interaction with the cells involved in the seroconversion of hepatitis b in the acute phase determines the stages of hepatitis B infection.
Expansion of myeloid-derived suppressor cells (MDSCs) makes the immune system unable to respond because as we saw above in those articles the immune system is it a reticular and syntrophic entirety, a single error can cause a series of wrong chain reactions, NK cells, DC cells, T cells, B cells not being able to sustain their function, partial or totally.
These chain errors allow the manifestation of chronic Hepatitis B in adults who have been infected at an early age, because we see clearly in healthy adults who come into contact with acute Hepatitis B the immune response is complete and the virus is seroconverted by the immune system. Depending on the age at which the infection occurs, the immune system has the ability to respond or not. The infection at an early age coincides with a high rate of chronicity and an infection at a young or adult age coincides with a functional resolution.
Current treatments of chronic Hepatitis B are very focused on blocking the virus but these treatments take years. The infection with the acute Hepatitis B virus is controlled by the immune system in a maximum period of 6 months, which shows us that if we succeed in a sustained answer by immune system we can hope for a shorter duration of treatment.
The virus itself seems to be easily controllable in adulthood by the immune system, but the mechanisms by which it actually manages to escape are difficult to control in babies and the only effective method of protection remains vaccination.
A true cure of HBV infection may not be feasible because HBV DNA is in the nucleus as the episomal form of cccDNA and integrated into the host genome producing HBsAg. Even among persons who have recovered from acute HBV infection, viral covalently closed circular DNA (cccDNA) can still be detected in the liver, explaining the reactivation of HBV replication when these “recovered” persons are profoundly immunosuppressed or in other clinical settings [63,64].
Unlike other viral infections such as HIV or Hepatitis C, in acute Hepatitis B in young people and adults in 95% of cases, if all the cells of the immune system work, the HBs antigen is eliminated from the blood and antibodies are created, but even after the acute infection there is a covalently closed circular DNA in the liver in the long term.
On the other hand, in babies the situation is opposite, the younger they got infection, the greater the chances of chronicity.
If a baby becomes infected through vertical transmission or in the first months of life, it will become chronic with the form of HBe positive antigen, respectively the form that is responsible for a high viral load and a high contagion.
We found that myeloid-derived suppressor cells (MDSCs), which is rarely seen in healthy adults, are present in large numbers in new-borns and their frequency decreases rapidly in the first months of life, this could explain HBe antigen tolerance in newborns.
Given the above articles, we can see that Hepatitis B does not induce a congenital antiviral response or intrahepatic immune responses in new-borns as long as myeloid-derived suppressor cells (MDSCs) are present in large numbers, because we are talking about the inability of the immune system to present antigen and respond, practically an ideal environment for the development of Hepatitis B.
Myeloid-derived suppressor cells (MDSCs) throughout immunosuppressive function facilitates pregnancy successfully, contributing to maternal fetal-tolerance but myeloid-derived suppressor cells (MDSCs) will be responsible for tolerance of hepatitis B by the immune system as well.
The first step in initiation of the T-Cell response is to bring antigens by DC in lymph nodes, lymph nodes representing the common meeting point of Antigens with T Cells and B Cells.
The first DC response is to internalize the HBs virus into the DC and degrade it into a peptide. Hepatitis B virus interacts with innate receptors TLR and the signal generated will activate the DC. After DC expresses the chemokine CCR7, migrates into lymph node.
Babies infected by vertical transmission have very high values of viral load (over >1000.000.000 U.I/ML) and also very high amounts of quantitative antigen which represent viral transcription in hepatocytes (over>100.000 U.I/ML), but without inflammation as long as mdsc are present in large numbers. This represents the immunotolerance phase when myeloid-derived suppressor cells (MDSCs) interact with chemokine CCR7.
When a DC doesn’t express chemokine CCR7, DC is retained in inflamed tissue and doesn’t migrate in the lymph node and in this manner the peptides from Antigens HBe and HBs are not presented to the T cells.
This is the reason why we do not see liver damage in babies despite the very high HBV DNA because myeloid-derived suppressor cells (MDSCs) are in large numbers.
This will allow tolerance to hepatitis B, immune system will protect the virus exactly as it maternal-fetal tolerance.
Hepatitis B does not cause direct damage to the liver, the immune system's response is responsible for this because T cells cannot enter in an infected cell, this function does not exist, T cells can only destroy a virus in a cell only by completely destroying the infected cell.
This is why we see high levels of liver enzymes (ALT/AST) in the manifestation of acute hepatitis in adults, because the immune system destroys infected cells.
The decrease in myeloid-derived suppressor cells (MDSCs) in the lymph node and tissue allows the immune system to respond, and this coincides with the immune system response confirmed by the decrease in viral load and the decrease in quantitative hbs antigen which is correlated with hepatitis B virus transcription in liver cells. T cytotoxic cell are responsible for this decrease.
If a child of 2 years or older becomes infected with Hepatitis B, we see a different situation, because the immune system seems to be able to eliminate the HBe antigen, we can correlate this with the fact that myeloid suppressor cells decrease with age and thus the immune system will tolerate a single antigen, namely the HBs antigen.
So, we are talking about a different situation of the immune system that practically allows the detection and elimination of HBe antigen.
Immune system response was complete against HBe antigen by two signals required for T-cells activation. Signal 1 is represented by Peptide HBe -MHC complex and signal 2 is represented by costimulatory molecule.
Most important co-stimulatory are B7-1(CD80) and B7-2 (CD86), which both bind to CD28.
MDSC interacts with these co-stimulatory molecules throughout immunosuppressive function of inhibitory protein CTLA-4 in lymph node and PD1 in tissue, which express a higher affinity than costimulatory molecules and thus maintains T cell exhaustion against hepatitis B.
Signal 1, respectively Peptide Antigen HBs -MHC Complex, by itself is not enough for T cell activation, it induces tolerance without the second required signal, namely co-stimulatory molecules.
The response of the adaptive system in hepatitis B starts when a B or T cell meets the antigen expressed by the DC cell. There are very few naive T cells for each Antigen, the role of naive T cells is to circulate and discover antigens in the lymph nodes. In the lymph nodes there is a special blood vessel called High Endothelium venule that allows T cells to bind to it because both have the right chemokine CCR7 receptors. Dendritic cells will express the same CCR7 molecule that will allow the interaction between T cells and DC cells in the interfollicular area of the lymph node.
These two signals Peptide Antigen HBs -MHC Complex and costimulatory molecules allow clonal expansion of CD8+ and allow their differentiation into CTL cells, which are effector cells. CTL cells contain granules that are effective against viral infected cells.
People with negative HBe antigen and undetectable viral load have restored partial control of the immune system. CTL cells control the viral load which shows us that there is no more inhibitory protein in lymph nodes but they continue to exist in the tissue/liver and thus maintain T cells exhaustion.
The presence of myeloid-derived suppressor cells (MDSCs) in the liver are responsible for maintaining the infection with hepatitis B by inhibiting costimulatory molecules. The inhibitory proteins generated by myeloid-derived suppressor cells (MDSCs) have higher affinity than the signals required for T cell activation and this coincides with tolerance.
Now, my abstract question is whether immune control cannot be restored in patients with Hepatitis B using drugs that inhibit myeloid suppressor-derived cells (MDSC Inhibitors) along with drugs that can block NTCP (Hepatitis B and D receptor). Together with Hepatitis B-specific antivirals (entecavir / tenofovir) and possibly the Pegasys +/-, the immune system of patients with Hepatitis B might respond in the same way as in healthy adults, because we are already talking about a possible combination of drugs that involve several factors involved in Hepatitis B. There are already drugs on the market that have an inhibitory effect on myeloid-derived suppressor cells (MDSCs) cells and do not have increased toxicity, example AMILORIDE, there are also natural components, icariin, cucurbitacin I, cucurbitacin B and curcumin that can inhibit suppression mechanisms of MDSC or may induce their maturation. There are already drugs on the market that can inhibit NTCP (Hepatitis B and Hepatitis D entry receptor) that have had promising results in clinical trials and have acceptable prices, example EZETIMBE. Such a study can be easily done.
The immune system must function perfectly and no longer tolerate this antigen HBs to hope for a high rate of seroconversion in patients with Hepatitis B. Recovery of T cells function is possible if myeloid-derived suppressor cells (MDSCs) are inhibited.
The presence of myeloid-derived suppressor cells (MDSCs) in new-borns makes Hepatitis B to become chronic over years with both antigens present in the blood, HBs and HBe, the immune system not being able to recognize the infection in the first years of life. The expansion of myeloid-derived suppressor cells (MDSCs) allows the organism to maintain the infection with Hepatitis B, although the patient reaches adult age, because myeloid-derived suppressor cells (MDSCs) is responsible for the disfunction of the immune system and not the virus itself.
There are dugs already available on the market that have the capacity to inhibit myeloid-derived suppressor cells (MDSCs). And further more potentials treatments that can block the entry receptor for Hepatitis B and drugs that stop the viral replication in the blood.
It might be that the drugs pointed are not the winning combination to eliminate the HBs antigen, but the ideas above could lead to a new path to test other drugs and tackle the issue from a holistic point of view, using the potential of the immune system.
Citation: Oprea CC (2021) Potential Approach of Hepatitis B by Inhibiting MDSC to Obtain Control of the Immune System. Intern Med. S6:001.
Received: 19-May-2021 Accepted: 02-Jun-2021 Published: 10-Jun-2021 , DOI: 10.35248/2165-8048.21.s6.001
Copyright: © 2021 Oprea CC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.