Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2019)Volume 7, Issue 1
Background and objective: There is growing interesting for edible marine algae species as a source for beneficial polysaccharides. Ulva lactuca is a widespread macroalga and highly consumed by humans and livestock because of its great nutritional values. Many studies showed that different extracts of U. lactuca possess nutritional and biological values. Ulvan is one of its structural water-soluble sulfated polysaccharides. The main objective of this study is preparing synbiotic yogurt by using ulvan polysaccharide by different percentage as a prebiotic ingredient and using probiotic starter bacteria in the manufacture of set synbiotic yogurt. Then study the quality properties of the resultant synbiotic yogurt.
Methodology: ulvan polysaccharide was extracted from Ulva lactuca using the methods of hot water-extraction and ethanol-precipitation. The synbiotic yogurt was prepared from fresh skim milk and ulvan polysaccharide as prebiotics, which was added by different concentrations: 1%, 2% and 4% (w/v). The mixtures were heated to 90°C for 10 min and immediately cooled to 39°-40°C; and inoculated with 3% of probiotic starter culture (containing of Lactobacillus acidophilus LA-5 Streptococcus thermophilus TH-4, and Bifidobacterium sp. Bb-12). The inoculated milks were distributed into 100 mL plastic cups and incubated at 40°C till complete coagulation. The synbiotic yogurt were transferred to the refrigerator at 7° ± 1°C and storage for 9 days; then the treatments were analyzed for the bacteriological, physical, chemical, and organoleptic assessments at fresh time and after 9 days of cold storage.
Results: The results showed that adding of 1-2% of the ulvan polysaccharide presented synbiotic yogurt with good chemical and physical properties, as well as stimulated the growth and activity of probiotic bacteria. But the addition of high percentage (4%) gave opposite results in terms of flavor, delayed fermentation and weak texture with syneresis also.
Conclusion: Therefore, it is recommended to use ulvan polysaccharide as prebiotic in the manufacture of synbiotic yogurt by adding 1-2%; and the flavor can be improved by adding any natural flavor to the synbiotic yogurt.
Synbiotic yogurt; Ulvan polysaccharide; Probiotic bacteria; Chemical gross; Physical attributes; Bacteriological properties; Organoleptic assessment
The relevance between the consumer desires, the technological progress in food processing and the presence of conclusive evidence on the role of foods in the prevention of some disease have created a magnificent opportunity to address many public health issues through nutrition and diets. Increasing interest in the selection of foods that can be strengthened the health has led to use of “functional foods” term, which are food products with special components possess beneficial and physiological effects for human health. The functional food concept includes both probiotics and prebiotics as popular topics [1].
Lactic acid bacteria (LAB) are dignified probiotics bacteria; their beneficial effects on the health of the host have been exceedingly recognized. As a result of their fermentation products and metabolic activities, LAB have many physiological functions, e.g., improved digestion and absorption, vitamin synthesis, reduction of gas distension, reduction of cholesterol, inhibition the growth of potential pathogens, and immunostimulation [2]. Thence, LAB is mainly used in the foods industries. Prior probiotic bacteria reach and colonize the host large intestine to accomplish their physiological functions, they must keep their viability during the following three phases: Industrial processing, Storage, and Mobility through the stomach and the small intestine [3].
1. Industrial processing,
2. Storage, and
3. Mobility through the stomach and the small intestine [3].
Prebiotics are non-digestible food ingredients and selectively used by host microorganisms to confer a health benefit; and stimulates the growth of beneficial bacteria in the gastrointestinal tract. Prebiotics usually exert beneficial effects on probiotic growth and the host health. The potential criterion for prebiotic ingredients is that they must cross through the large intestine without being absorbed or digested in the upper GI tract; consequently, they become accessible for probiotic bacteria. Prebiotics effects are associated with positive changes in the composition of GI microbiota. The important activity for prebiotic substances is the significant increase in the viable counts of bifidobacteria and lactobacilli. These two genera have wide activities; bifidobacteria have been shown to restore the normal flora after antibiotic therapy, stimulate the immune system, produce vitamin B and inhibit the growth of the pathogen. Whereas, lactobacilli help lactose-intolerant persons to digest lactose, as well as reduce diarrhea, constipation or irritable bowel syndrome. Moreover, prebiotics reduced the incidence and duration of intestinal infections, improve the digestion, downregulate the allergic response, and improve the elimination of stool [4].
Strengthening the growth medium with prebiotics is an effective way for improving the proliferation of Probiotic LAB and promoting their viability under challenging conditions. Probiotic LAB strains can use different prebiotic as a type of carbohydrates to produce the required energy for their proliferation. Moreover, carbohydrates contain numerous hydroxyl groups; which they can form hydrogen bonds with polar groups on proteins to increase protein stability, which could enhance the cell survival. Prebiotic carbohydrates are mainly polysaccharides, oligosaccharides, protein hydrolyzates, and plant extracts. Previous studies showed that some plant polysaccharides could be used as prebiotics; such as ginseng polysaccharides, mushroom polysaccharides, mango fibrous polysaccharides, and algal polysaccharides [3].
Green algae or seaweeds are one of the three main types of marine macroalgae, along with the red and brown seaweeds, which can synthesize sulfated polysaccharides. These polysaccharides have an important role in the structural of green seaweeds; they located in the fibrillar matrix and intercellular space of the cell wall where they bind with proteins, cellulose and other polysaccharides forms by ionic interactions and hydrogen bonds. Ulva lactuca (family: Ulvaceae), which also known as the sea lettuce; is a widespread macroalga in the intertidal zone, protected and calm seaports. This seaweed is highly consumed by humans and livestock because of its considerable nutritional values. Contemporary studies showed that different extracts of U. lactuca possess nutritional and biological values. Ulvan is one of its structural water-soluble sulfated polysaccharides; the structure of ulvan complex is varied, depending on the species of algae, places and conditions of cultivation, and extraction methods [5]. Ulvan is highly charged sulfated polyelectrolytes, mainly consisting of rhamnose, xylose, and uronic acid as the basic monomer sugars, and also containing disaccharide. The ulvans cell-wall polysaccharides represent 38-54% of the dry matter of algal [6]. They are rich in hydroxyl (-OH) groups, which make them hydrophilic and water-soluble; and establish rigid and stiff intra-chain H-bond networks, so they considering good thickeners. Their regularity structures also boost interactions with external ions and inter-chain H-bonding (e.g., gelation). In the same way, their gelling and rheological properties make them proper as a substitute for gelatin and related compounds in some foods [7].
Ulvans are not degraded by the enzymes of the upper part of GIT and serve as a great source for dietary fibers; which present technological features when used as ingredients in the food products. These dietary fibers vary in their physical and chemical properties than that in most vegetables and fruits. Their prebiotic properties also predetermine the perspective of using them as a source of necessary nutrition for the growth of probiotic lactic acid bacteria. Furthermore, these algal polysaccharides possess unique fermenting and biochemical properties, such as immunostimulating, antibacterial, anticancer, antiviral, and other biological benefits [8]. Thence, considerable attention is paid for using ulvan as emergent prebiotics that possesses a significant possibility to be used as functional food ingredients or nutriceuticals utilized directly in food or feed or administered as pills [7].
The synbiotic products are products comprising of both probiotics’ bacteria and prebiotic ingredients. Yogurt and fermented milk products have important nutritional properties and presenting a favorable environment for the survival and growth of probiotic bacteria. The objectives of the present study are the evaluation of the efficiency of using ulvan polysaccharide as prebiotics by different concentrations, with the combination of probiotic bacteria in the manufacturing of synbiotic set yogurt. Then, study the physiochemical, rheological, microbiological and sensory features of the resultant synbiotic yogurt.
Materials
Fresh raw buffaloes’ milk was provided by the farm of Faculty of Agriculture, Ain Shams University. Ulva lactuca was obtained from the department of microbiology and phycology, Faculty of Science, Zagazig University, Egypt. Skim milk powder (97% total solids) was purchased from Dina farm, Sadat city, Egypt.
Commercial ABT-7 freeze-dried DVS mixed starter of thermophilic lactic acid strains with potential probiotic properties (containing of Lactobacillus acidophilus LA-5 Streptococcus thermophilus TH-4, and Bifidobacterium sp. Bb-12) was obtained from Chr. Hansen Laboratory, Copenhagen, Denmark. The bacteriological media M17 agar and MRS agar were obtained from Merck, Germany. Lithium and L-cysteine chloride were purchased from Sigma Chemical CO., USA.
Methods
Extraction of ulvan polysaccharide from Ulva lactuca aqueous extract: Fresh Ulva lactuca samples were obtained during spring of 2018 from The Abu Qir coast, Mediterranean Sea, Alexandria, Egypt. The samples were washed and cleaned with a diluted sodium chloride solution to remove any contaminants. The cleaned seaweed samples were dried in an oven at 50°C. The dried samples were crushed, ground in an electric blender to form a powder, and stored in the refrigerator as recorded by Tran et al. [5].
Ulvan polysaccharide was extracted by the modified procedure of Hussein et al. [6] using the methods of hot water-extraction and ethanol-precipitation. 100 g of dried Ulva lactuca were extracted three times at 100°C for 2 h with double-distilled water. The collected solution was centrifuged at room temperature for 15 minutes at 6000 rpm; then the extract was precipitated by ethanol (4-times the used volume of aqueous extract), and this mixture was kept at 4°C overnight. The precipitate was collected by centrifugation at 6000 rpm for 15 min and then dissolved in distilled water to dialyze against deionized water for 72 h for removing any traces for the alcohol. Finally, the precipitate was freeze-drying to yield the crude ulvan polysaccharide which its touch and appearance were like a brown gel.
The yield of polysaccharide (%) was calculated by the following equation:
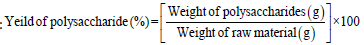
Chemical analysis and viscosity of ulvan polysaccharide: Total carbohydrates were measured spectrophotometrically at 490nm by the phenol-sulfuric acid procedure using D-glucose as a standard [9]. Sulfate content was measured as described by Sullivan [10] using Ion Chromatography (ICS-1100-Thermo Dionex, USA). The viscosity of ulvan solution (1%) was elucidated by Ostwald’s viscometer according to the method of Suresha et al. [11]. and the obtained values were expressed as millipascal in second (mPa.s).
The manufacture of synbiotic yogurt: The synbiotic yogurt treatments were prepared by the described steps of Al-Sheraji et al. [12]. Fresh Buffalos’ milk was separated mechanically to skim milk (0.3% fat and 9.5% solids not fat), and the milk was standardized to 12% total solids using skim milk powder. The synbiotic yogurt was prepared from fresh skim milk and ulvan polysaccharide as prebiotics, which was added by different concentrations: 1%, 2% and 4% (w/v). The mixtures were heated to 90°C for 10 min and immediately cooled to 39°-40°C; and inoculated with the starter culture (3%). The inoculated milks were distributed into 100 mL plastic cups and incubated at 40°C till complete coagulation (pH 4.7 ± 0.1); the incubation time for each treatment was recorded by using stop watch. The synbiotic yogurt was transferred to the refrigerator at 7° ± 1°C and storage for 9 days, and the treatments were as follow:
• T1: Synbiotic yogurt enhanced with 1% ulvan polysaccharide.
• T2: Synbiotic yogurt enhanced with 2% ulvan polysaccharide.
• T3: Synbiotic yogurt enhanced with 4% ulvan polysaccharide.
Three replicates for every treatment were done and the samples were analyzed microbiologically, sensory, chemically, and rheology when fresh and after 9 days of cold storage (7° ± 1°C).
Chemical analysis of synbiotic yogurt: Total solids, lactose, ash, protein contents and acidity (expressed as grams of lactic acid/100 g of sample) were estimated in the synbiotic yogurt samples by the recorded procedures in AOAC [13]. While the pH values of the samples were recorded using a digital pH meter (HI 93 1400, Hanna Instruments, Italy).
Bacteriological analyses of synbiotic yogurt: The synbiotic yogurt samples were prepared for bacteriological assessments based on the described method of Wehr and Frank, [14]. The enumeration of the viable cells of Lb. acidophilus was carried out on MRS-sorbitol agar at 37°C/72 h (incubation in an anaerobic environment using BBL Gas Pak, Becton Dickinson Microbiology Systems). The counting of bifidobacteria was done on MRS agar supplemented with 0.3% lithium chloride and 0.05% L-cysteine at 37°C/72 h (incubation in an anaerobic environment using BBL Gas Pak, Becton Dickinson Microbiology Systems). M17 agar was used to count the viable cell of Str. Thermophilus aerobically at 37°C/48 h. All the obtained results were expressed as log10 colony forming unit (cfu)/g of sample.
Physical and texture analysis of synbiotic yogurt: The apparent viscosity of synbiotic yogurt was measured at 20°C for fresh and after 9 days of cold stored (7° ± 1°C) samples; using a Brookfield DV-E viscometer (Brookfield Engineering Laboratory Inc., Stoughton, MA) as formerly reported by Djurdjevic et al. [15]. The Wingather software (Brookfield Engineering Laboratory Inc., Stoughton, MA) was used to collect the obtained data; which expressed as Pascal in second (Pa.s). The Firmness features of synbiotic yogurt samples were measured by the penetrometer (Kochler co. Inc., USA) in fresh time and after 9 days of cold storage (7° ± 1°C) as described by Amatayakul et al. [16]. The depth of penetration was adjusted at 10 mm and the speed of penetration was 2 mm/s.
The syneresis rate of synbiotic yogurt treatments was estimated as the described steps by Han et al. [17]. Twenty grams of yogurt sample was centrifuged in in centrifuge cups at 350 × g (model K-24; Sigma Laborzentrifugen GmbH, Germany) for at 4°C for 10 min. The resultant supernatant was collected to weight, and then syneresis index was calculated by the following equation:
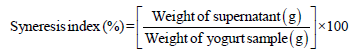
Organoleptic assessment of synbiotic yogurt: The synbiotic yogurt samples were assayed for the main organoleptic attributes in the fresh time. Eight panelists from the staff members of Food Science Department, Faculty of Agriculture, Ain Shams University, were asked to score the treatments for consistency and texture (30 points), flavor (60 points), and appearance (10 points) by using the described scheme by Salem et al. [18].
Statistical analysis
The obtained data of the experiments were presented as mean values. All the statistical analysis was done using the SPSS 16.0 Syntax Reference Guide [19] and the statistically different groups (p ≤ 0.05) were determined by the least significant difference (LSD) test.
Chemical properties and viscosity of ulvan polysaccharide
Ulvan complex is sulfated heteropolysaccharide presented in the cell wall of green seaweeds Ulva sp. Ulvan introduces a prospect source of novel functional biopolymer due to its particular structure and composition [20]. The composition of sugar in ulvan is greatly changeable but xylose, rhamnose, iduronic and glucuronic acid, and the existence of sulfate groups have been reported as the basic compounds of the polymer [21].
The chemical properties of obtained ulvan polysaccharide from Ulva lactuca green seaweed by hot water extraction method are presented in Table 1. The yield of polysaccharide was 10% of algal dry weight. This result of yield in the previous range of Lahaye and Robic [20] who mentioned that the range of yield was 8-29% of the algal dry weight, relying on the method of extraction and processing of purification. In the same time, this obtained value was higher than that obtained by Siddhanta et al. [22], who reported that the yield of polysaccharides from Ulva lactuca was 6.3% by the hot water extraction method. Whereas, the recorded yield by Mervat et al. [23] was 14.83% from the same algae and by the same extraction method. Table 1 also showed the sulfate content was 3.998% of ulvan dry weight. This result is lower than the recorded values in previous studies, and this may be due to the different methods which were used in the determination of sulfate. The total carbohydrates content was 26.41% in the obtained ulvan. This result is higher than that obtained by He et al. [24], and lower than Siddhanta et al. [22], which was 23.71% and 47.4%, respectively. The obtained results for sulfate and carbohydrates are in the same trend of Jaulneau et al. [25] who found that the ethanol-soluble ulvan had low quantities of sulfate and neutral sugars.
| Yield (%) | Sulfate (%) | Total carbohydrates (%) | Viscosity (1% solution of ulvan) |
|---|---|---|---|
| 10 | 3.998% | 26.41 | 18 mPa.s |
Where: mPa.s: milli Pascal in second.
Table 1: Chemical properties and viscosity of ulvan polysaccharide.
The obtained results for the chemical features of prepared ulvan in this study are in agreement and disagreements with the previous researches. These variations can be referred to the differences in the composition and structure of prepared ulvan polysaccharides; which vary, depending on the algae species and age, geographic location and season of cultivation, and the method of extraction. Particularly, in edible species U. lactuca, the results of published studies confirmed that the structural composition of ulvan polysaccharides is changeable and complex [5-7].
The viscosity of ulvan solution (1% in distilled water) was 18 mPa as shown in Table 1; this result was higher than obtained by Siddhanta et al. [22], who found that the viscosity was 18 mPa in 1.6% ulvan solution. The result of viscosity is analogous to that with Yaich et al. [26] who explained that ulvan solution appeared a shear-thinning fluid showing pseudoplastic behavior. Whereas, Robic et al. [21] mentioned that ulvan solution is displayed a bead-like structure, slightly linked by filaments. Gordillo et al. [27] also reported that high sulfate integration in ulvan polysaccharide may forms crosslink, which relays on interand intra-molecular crosslink, which is obstructed by highly negative groups, like sulfate groups, carboxylic acids in uronic acids, and/or methyl groups particularly in rhamnose. The gel formation of ulvan polysaccharide is complex, unique, and not fully understood yet. When it compared to arabic gum; ulvan gel introduced different functional properties than that gum; it was thermoreversible without thermal hysteresis. These gelling merits can be useful in the applications that gel formation is needed to be carefully controlled [23].
Total time for full coagulation of synbiotic yogurt enhanced with ulvan polysaccharide
The manufacture of yogurt is finished when the fermentation process is completed, which known by full coagulation of yogurt milk and the pH value reached to 4.6 ± 0.1. The needed time for full coagulation of synbiotic yogurt enhanced with ulvan polysaccharide or to reach the desired pH is shown in Table 2. The obtained times indicated that the enhancement yogurt with 1 and 2% of ulvan polysaccharide enhanced the growth of starter bacteria to produce lactic acid from lactose and finish the fermentation process in short time than the other treatments. While, the addition of high percent of ulvan polysaccharide (4%) had an adverse effect on the starter bacteria, which this treatment fermented in longest time than the others (260 minutes). These results confirmed that ulvan polysaccharide in low concentration had a significant (p ≤ 0.05) effect in the reduction of incubation time in the synbiotic yogurt manufacture. These results in the same line with that of Eskandari et al. [28] who reported that the supplementation of yogurt milk with 0.2% of sucrose, tryptone, or yeast extract has a significant effect in the reduction of incubation time.
| Treatments | Time (minutes) |
|---|---|
| Control | 230b |
| T1 | 205c |
| T2 | 180c |
| T3 | 260a |
Where: a, b,c Means within the same column with different superscripts are significantly different (p £ 0.05). T1: Synbiotic yogurt enhanced with 1% ulvan polysaccharide, T2: Synbiotic yogurt enhanced with 2% ulvan polysaccharide, T3: Synbiotic yogurt enhanced with 4% ulvan polysaccharide.
Table 2: Total time for full coagulation for synbiotic yogurt treatments.
Gross composition of synbiotic yogurt enhanced with ulvan polysaccharide
There is growing interesting for edible marine algae species as a source for gorgeous polysaccharides. Ulvan polysaccharide is one of these polysaccharides, which can introduce set yogurt with considerable chemical, structure, and microbiological properties. Table 3 summarized the obtained results for some of the chemical attributes of synbiotic yogurt samples. Synbiotic yogurt enhanced with a different percent of ulvan polysaccharide were higher in total solids, protein, and ash contents than the control; these contents were increased by increasing the addition of polysaccharide and only significant (p ≤ 0.05) in 4% ulvan polysaccharide-enhanced treatment. There are many reasons for this increase in some chemical composition; one of them is using a high temperature for a long time during the manufacturing of yogurt possibly increased chemical composition. Another reason is that the used ulvan polysaccharide contained different components which contributed in total solids of the resultant yogurt. The obtained results were in agreement with Al-Sheraji et al. [12] who found that the addition of polysaccharide from mango peel increased the total solids of non-fat yogurt. Hussein et al. [6] also reported that the enhanced yogurt with polysaccharide of Jew’s-mallow had total solids content higher than control. By the same way, Debon et al. [29] mentioned that the total solids of fermented milk were increased by increasing the level of oligofructose and inulin. Also, Villegas et al. [30] found that the total solids of low-fat milk beverages were increased by increasing the added percent of inulin.
| Treatments | Total solid | Protein | Lactose | Ash |
|---|---|---|---|---|
| Control | 13.11b | 4.95c | 3.35b | 0.78b |
| T1 | 13.60ab | 5.22bc | 3.21b | 0.92b |
| T2 | 13.92a | 5.51ab | 3.05b | 1.26b |
| T3 | 14.21a | 5.86a | 3.94a | 1.94a |
Where: a, b,c Means within the same column with different superscripts are significantly different (p £ 0.05), T1: Synbiotic yogurt enhanced with 1% ulvan polysaccharide, T2: Synbiotic yogurt enhanced with 2% ulvan polysaccharide, T3: Synbiotic yogurt enhanced with 4% ulvan polysaccharide.
Table 3: Chemical composition (%) of synbiotic yogurt enhanced with ulvan polysaccharide.
Lactose content of synbiotic yogurt enhanced with 1 and 2% of ulvan polysaccharide was lower than control and yogurt enhanced with 4% of ulvan polysaccharide as seen in Table 3. These results showed the stimulatory ability of ulvan polysaccharide until 2% on the growth and activity of starter bacteria; but the addition of high percentage of ulvan polysaccharide had a slight inhibition effect on the growth of starter culture. In high enhancement treatment (4% ulvan polysaccharide), the lactose content and the time for coagulation were significantly (P ≤ 0.05) highest than the others; these results can be more explained by the obtained results of viable counts of starter bacteria, pH and acidity values.
The changes of acidity and pH values of synbiotic yogurt enhanced with ulvan polysaccharide
Changes in the pH and acidity values in synbiotic yogurt treatments at fresh time and after 9 days of cold storage (7° ± 1°C) were presented in Table 4. The enhancement with 1 and 2% ulvan polysaccharide significantly (P ≤ 0.05) reduced the pH and increased the acidity values for treated yogurt than control and 4% ulvan polysaccharide-enhanced yogurt. These results can be explained by the addition of ulvan polysaccharide (1 and 2%) can act as good prebiotics for the starter and probiotic bacteria. This interpretation is also consistent with previous results of Elbanna et al. [31] who reported that the expolysccharides was act as prebiotic ingredient for the lactic acid bacteria in low-fat yogurt. By the same way, Salazar et al. [32] mentioned that oligosaccharides from exoplosccharides are consumed by Lactobacillus bacteria, so their activities increased. Moreover, Al-Sheraji et al. [12] assumed that the addition of 0.05 and 0.1% mango polysaccharide increased the acidity of free fat yogurt.
| Treatments | pH | Acidity (as lactic acid %) | |||
|---|---|---|---|---|---|
| Storage period (days) | |||||
| Fresh | 9 | Fresh | 9 | ||
| Control | 4.61Aa | 4.53Ba | 0.91Ya | 1.10Zb | |
| T1 | 4.58Aab | 4.45Bb | 0.95Ya | 1.24Za | |
| T2 | 4.52Ab | 4.42Bb | 0.98Ya | 1.33Za | |
| T3 | 4.64Aa | 4.56Ba | 0.78Yb | 0.91Zc | |
Where: A, B Means within the same row with different superscripts are significantly different (p £ 0.05), Y, Z Means within the same row with different superscripts are significantly different (p £ 0.05), a, b,c.. Means within the same column with different superscripts are significantly different (p £ 0.05), T1: Synbiotic yogurt enhanced with 1% ulvan polysaccharide, T2: Synbiotic yogurt enhanced with 2% ulvan polysaccharide, T3: Synbiotic yogurt enhanced with 4% ulvan polysaccharide.
Table 4: pH values and acidity (%) of synbiotic yogurt enhanced with ulvan polysaccharide during cold storage (7° ± 1°C).
The obtained results of pH and acidity values for 4% ulvan polysaccharide-enhanced yogurt were different than that synbiotic yogurt enhanced with 1 and 2% ulvan polysaccharide. This difference can be explained by the fact that the increasing percent of ulvan polysaccharide in synbiotic yogurt may be slightly inhibiting the activity of lactic acid bacteria; and the previous result for lactose content and fermentation time were appropriate with that explanation. These results in agreements with Al-Sheraji et al. [12] who concluded that the acidity values of free fat yogurt enhanced with 0.15% mango polysaccharide was lower than that enhanced with 0.05 and 0.1% mango polysaccharide.
During the cold storage (7° ± 1°C) the acidity gradually increased, and pH values decreased significantly (p ≤ 0.05), this can be due to the continuous of lactose hydrolysis and acids formation as a result of starter culture activity. These results in the same trend of Gustaw et al. [33] who found yogurt enhanced with 1% inulin had the lowest pH values during the refrigeration storage. Ehsani et al. [34] also mentioned that the prebiotic such as lactulose, inulin and oligofructose stimulate the activity and growth of probiotic bacteria and consequently its acids production, which increased the acidity and reduced the pH values in the resultant yogurt during the storage time. On the other hand, Moayednia [35] found a significant decrease in the pH value after 8 days of cold storage of yogurt; and reported that results were logical because the β-galactosidase of viable or nonviable cells of starter culture was still active.
Bacteriological enumeration of synbiotic yogurt enhanced with ulvan polysaccharide
The important factor for the dairy products containing probiotic bacteria particularly the yogurt which is an acidic environment is the survival counts of the used probiotic bacteria. There are many factors affecting their survivability in yogurt such as the probiotic strains, inoculation percentage, conditions of culture growth, fermentation time and temperature, and the acidity rate in yogurt [34].
The changes of viable counts of Streptococcus thermophiles, Bifidobacterium sp., and Lactobacillus acidophilus in synbiotic yogurt enhanced with different percentages of ulvan polysaccharide during the cold storage (7° ± 1°C) for 9 days are shown in Table 5. Streptococcus thermophilus is a sole fermenting strain, so its counts were higher than those of Lactobacillus acidophilus, and Bifidobacterium sp. in all treatments. In the same time, the obtained counts for probiotic bacteria were more than the lowest recommended therapeutic level (106 cfu/g); whether at the beginning or end of the storage period. These findings are analogous to that mentioned by Gustaw et al. [33] and Eskandari et al. [28].
| Storage period (days) | Treatments | |||
|---|---|---|---|---|
| Streptococcus thermophilus | ||||
| Control | T1 | T2 | T3 | |
| Fresh | 8.3Ab | 9.2Aa | 9.5Aa | 7.9Ab |
| 9 | 6.8Bb | 7.6Ba | 8.1Ba | 6.2Bb |
| Lactobacillus acidophilus | ||||
| Fresh | 7.5Ab | 8.3Aa | 8.4Aa | 7.2Ab |
| 9 | 6.1Bb | 7.0Ba | 7.0Ba | 5.9Bb |
| Bifidobacterium sp. | ||||
| Fresh | 7.0Ab | 7.6Aab | 7.9Aa | 7.1Ab |
| 9 | 5.8Bb | 6.4Aab | 6.8Ba | 6.0Bb |
Where: cfu: Colony forming unit. A, B Means within the same column with different superscripts are significantly different (p £ 0.05), a, b,c.. Means within the same row with different superscripts are significantly different (p £ 0.05), T1: Synbiotic yogurt enhanced with 1% ulvan polysaccharide, T2: Synbiotic yogurt enhanced with 2% ulvan polysaccharide, T3: Synbiotic yogurt enhanced with 4% ulvan polysaccharide.
Table 5: The counts of viable cell (log10 cfu/ml) of starter bacteria of synbiotic yogurt enhanced with ulvan polysaccharide during cold storage (7° ± 1°C).
Table 5 also showed that the counts of Lactobacillus acidophilus were higher than Bifidobacterium sp. in all samples; that may be due to the associative behavior of Lactobacillus acidophilus and Bifidobacterium sp. bacteria. The growing of Bifidobacterium sp. is slow in milk, because of its slight proteolysis activity and deficiency of non-protein nitrogen (NPN) in milk, which makes milk is an inappropriate medium for growth of Bifidobacterium sp. and coexistence with Lactobacillus acidophilus enhanced its growth [34].
The results also showed that the counts of all the starter bacteria were significantly (p ≤ 0.05) higher in synbiotic enhanced with 1 and 2% ulvan polysaccharide than the control and 4% ulvan polysaccharideenhanced yogurt either at the fresh time or after 9 days of cold storage. The viable counts of Lactobacillus acidophilus and Bifidobacterium sp. in synbiotic yogurt increased in enhanced samples with 1 and 2% ulvan polysaccharide; which confirmed the synergistic effect between these bacteria due to their mutual interactions and the addition of ulvan polysaccharide as a prebiotic. These results are compatible with that demonstrated before by Gustaw et al. [33] and Ehsani et al. [34] who confirmed that the incorporation of inulin, oligofructose, and other types of fibers as prebiotics improved the growth of, Bifidobacterium sp. and Lactobacillus acidophilus probiotic bacteria. Furthermore, these results showed that ulvan polysaccharide at the concentrations of 1-2% are beneficial and exhibited stimulatory effects on the activity and growth of Lactobacillus acidophilus and Bifidobacterium sp. in the resultant synbiotic yogurt. While, the synbiotic enhanced with 4% ulvan polysaccharide has lowest counts of all the starter strains, which that meaning the addition of a high amount of this polysaccharide had a little inhibitory effect on the growth and activity of these bacteria in the resultant synbiotic yogurt. These observations are in the same line of Gustaw et al. [33] who concluded that the addition of 1% oligofructose was useful for Bifidobacterium sp. and Lactobacillus acidophilus; but the higher concentrations (2 and 3%) decreased significantly all the bacterial counts during the cold storage time. Moreover, there is a significant reduction in the counts of probiotic bacteria in control samples; these reductions may be due to the high level of acids formed by starter bacteria and the absence of prebiotics as enhancing growth agents.
In the end of cold storage (7° ± 1°C) all the counts of bacteria were significantly (p ≤ 0.05) decreased. The further decreasing of pH during post-acidification caused these reductions in the viability of probiotic bacteria in synbiotic yogurt; also, Bifidobacterium was more susceptible to aerobic conditions during the cold storage. These findings are consonant with that observed by Eskandari et al. [28] Gustaw et al. [33], and Ehsani et al. [34].
Physical and texture analysis of synbiotic yogurt enhanced with ulvan polysaccharide
The texture is one of the most important properties that clarify the quality of set yogurt. The most known defects related to the texture of yogurt and cause consumer rejection are variations of apparent viscosity and the liberation of whey or the syneresis. The texture of yogurt and some dairy products can be controlled by using hydrocolloids, which can interact with the casein network. The functional role of hydrocolloids with low percentages is enhancement the viscosity and/or reduction of syneresis. Both hydrocolloids (basically polysaccharides) and proteins boost the structural properties of the yogurt, depended on their ability to confer structure to the continuous phase of the medium [36].
Apparent viscosity: Figure 1 illustrated the changes of viscosity for all synbiotic yogurt samples. The obtained values showed that the addition of 1 and 2% ulvan polysaccharide significantly (P ≤ 0.05) increased the viscosity of synbiotic yogurt than the control and 4% ulvan polysaccharide-enhanced treatment. The additions of polysaccharide increment the viscosity due to the interactions between the polysaccharides and milk proteins. This finding was mentioned before by Zhang et al. [37] who cleared that the increase in viscosity of enhanced fermented skim milk may be due to the interaction between exopolysaccharides and milk proteins. In other words, the sugar and protein compounds of the milk interacted to compose a firm network and thse free water was reduced, so the viscosity of enhanced fermented skim milk was significantly increased. These presented results are analog to that reported by Hussein et al. [36] who found that yogurt enhanced with taro polysaccharide had the highest viscosity than the control. Gustaw et al. [33] also confirmed that addition of 1% fructooligosaccharides significantly increased the apparent viscosity of bio-yogurt than the control one; and explained that fructooligosaccharides being part of the structural network that formed during structuring and fermentation of bio-yogurt. Furthermore, Al- Sheraji et al. [12] indicated that the addition of mango polysaccharides resulted in non-fat yogurt with significantly higher viscosity than the control yogurt.
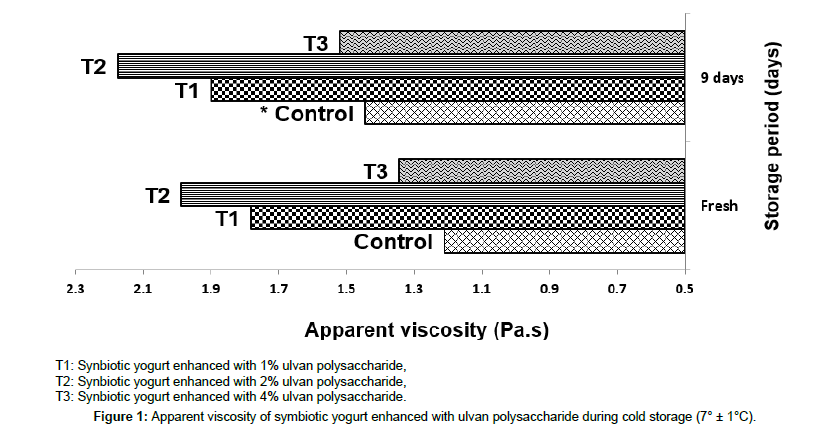
Figure 1. Apparent viscosity of symbiotic yogurt enhanced with ulvan polysaccharide during cold storage (7° ± 1°C).
On the contrary trend, the viscosity of synbiotic enhanced with 4% ulvan polysaccharide was lower than synbiotic yogurt enhanced with 1 and 2% ulvan polysaccharide and higher than control treatment; although it contained a higher percentage of total solids. This result may be due to that treatment consumed a long time in the fermentation Table 2, resulted in a weak texture and structure in the resultant yogurt.
Figure 1 also presented that the viscosity values for all treatments were insignificantly (p<0.05) increased during the storage period; this increase can be due to the hydration as mentioned before by Al-Sheraji et al. [12]. Meanwhile, these obtained results in the same trend of Gustaw et al. [33] and Hassan et al. [38].
Firmness: The penetrometer readings of synbiotic yogurt samples are shown in Figure 2. Higher penetrometer readings referred to higher firmness values. The penetrometer values significant increased (p ≤ 0.05) in the treated synbiotic yogurt with 1 and 2% ulvan polysaccharide than both of the control and 4%ulvan polysaccharide-enhanced treatment until the end of storage period (9 days). This means that the enhancement of synbiotic with 1-2% ulvan polysaccharide provided an increase in gel strength and firmness of resultant synbiotic yogurt than the other treatments. These findings are in accordance with Guzel-Seydim et al. [39] who reported that the generated exopolysaccharide from lactic acid bacteria could promote the texture of yogurt, by interacting with free water and reducing it in yogurt. In the same trend, Elbanna et al. [31] confirmed that the addition of 0.4-0.8% exopolysaccharide improved the firmness of lowfat yogurt. Gustaw et al. [33] also mentioned that the addition of 1% fructooligosaccharides significantly increased the hardness of bioyogurt than the control treatment. While, Han et al. [17] assumed that the highest concentration of exopolysaccharide compatible with the highest firmness and viscosity.
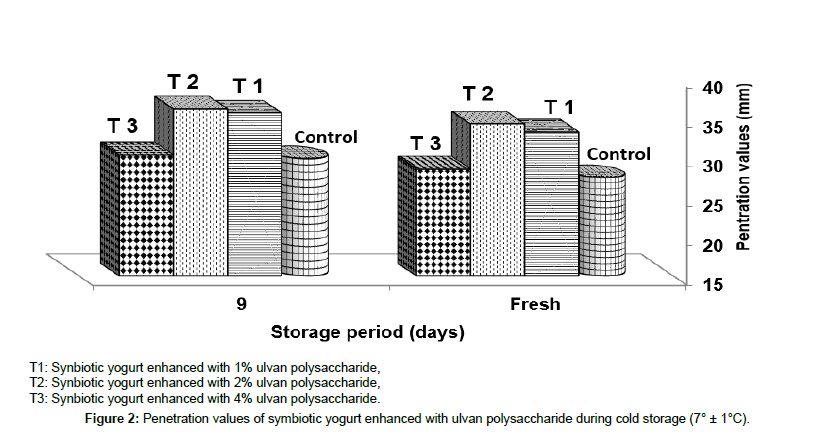
Figure 2. Penetration values of symbiotic yogurt enhanced with ulvan polysaccharide during cold storage (7° ± 1°C).
It can be seen in the same Figure 2 the synbiotic yogurt enhanced with 4% ulvan polysaccharide had lower firmness than the other enhanced treatments (T1 and T2) but in the same time its firmness higher than control treatment. This can be due to the long time of fermentation which reduced the firmness of synbiotic yogurt. This observation is in consistency with that mentioned by Han et al. [17] who recorded there is no linear correlation between the used concentrations of polysaccharides and the texture of resultant yogurt.
Otherwise, the firmness of all treatments was insignificantly (p<0.05) increased during cold storage (7° ± 1°C). These results are compatible with that of Gustaw et al. [33] and Hassan et al. [38].
Syneresis: Syneresis is a common flaw during the cold storage of yogurt, and the manufacturers try to minimize it as possible. Figure 3 presented the syneresis rate (%) for synbiotic enhanced with ulvan polysaccharide during the storage time (7° ± 1°C). Figure 3 showed that enhancement synbiotic yogurt with 1 and 2% ulvan polysaccharide resulted in products with significant (p ≤ 0.05) lower syneresis rate than both control and 4% ulvan polysaccharide-enhanced treatment until the end of storage period (9 days). Decreasing of the syneresis rate of the synbiotic yogurt enhanced with polysaccharides pointed to that the polysaccharides-milk protein network was formed through the whole synbiotic yogurt [12] and these results are like that obtained by many researchers. Amatayakul et al. [16] and Elbanna et al. [31] assumed that the low-fat yogurt fortified with exopolysaccharide had lower amount of whey (syneresis) existent on the surface of yogurt compared to the control treatment; and explained that the exopolysaccharide could strengthen the protein network by improving the free water binding functions. In the same trend Han et al. [17] mentioned that yogurt with the highest exopolysaccharide content is the lowest whey liberation. Moreover, Ehsani et al. [34] found that enhanced yogurt with lactulose as a prebiotic had significantly lower syneresis rate all over the storage period; and clarified these results that these prebiotics are soluble fibers which are known as water binding and structuring agents and could reduce the syneresis by raise the water-binding strength. Al-Sheraji et al. [12] confirmed that the addition of mango polysaccharides significantly decreased the syneresis of non-fat yogurt.
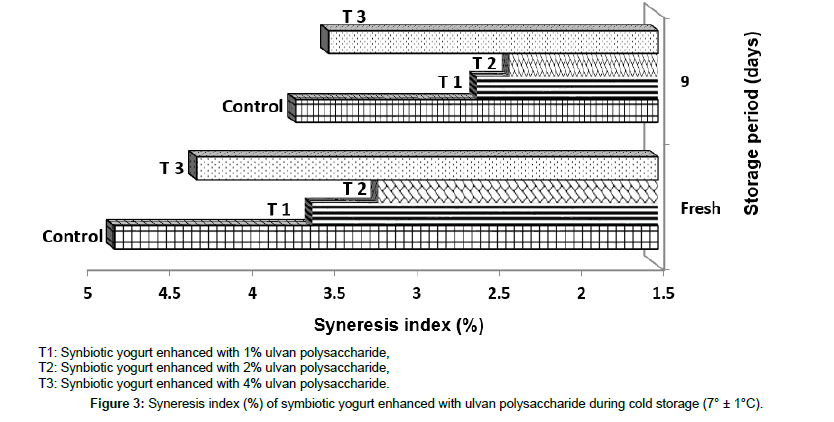
Figure 3. Syneresis index (%) of symbiotic yogurt enhanced with ulvan polysaccharide during cold storage (7° ± 1°C).
It is known if the acidification is fast, the syneresis will be high as mentioned by Lucey [40], but in this research the addition of ulvan polysaccharide (1-2%) increased the rate of acidification (Table 2) and decreased the syneresis rate. On the contrary, the obtained result of synbiotic yogurt enhanced with the highest percentage of ulvan polysaccharide (4%) was lowest acidification rate and highest syneresis rate than the other enhanced yogurt (1-2%).
In Figure 3 also, it can be seen that the syneresis rate in all treatments decreased by the end of storage time (9 days); and these results are congruent with that of Al-Kadamany et al. [41] who recorded that the separated whey in acid milk gels is linked by rearrangement the particles of the casein gel network.
From all the results of physical and viscosity of synbiotic yogurt, it is important to say that the addition of ulvan polysaccharide (1- 2%) as prebiotics improved the rheological properties of the resultant synbiotic yogurt by increasing the viscosity and firmness and reducing the syneresis rate.
Organoleptic characteristics of synbiotic yogurt enhanced with ulvan polysaccharide
The success of a yogurt product is depending on if it meets the requirements of the consumer and the degree of acceptance and satisfaction. The organoleptic assessment was achieved by the panelists who used a scoring scheme containing the specific sensory characteristics of the product acceptance for all the samples. Its worthy mention, the yogurt texture should be smooth, fixed gel, and free from whey liberation; while the appearance should be homogenous with a uniform white color.
The given scores for sensory assessment of synbiotic yogurt are illustrated in Table 6. The scores presented that the enhancement of yogurt with ulvan polysaccharide was acceptable in all treatments more than control. The lowest percentages of enhancement (1-2%) by ulvan polysaccharide were more accepted than the highest addition of this polysaccharide (4%) in all tested properties. From the flavor scores, it can be seen that by increasing the percent of added polysaccharide the given score was decreased and only significant (p ≤ 0.05) in the highest used percentage of ulvan polysaccharide in synbiotic yogurt (T3). These results may be due to the very slight like salty flavor which appeared in the higher treated samples (T2 and T3).
| Sensory attributes | Treatments | |||
|---|---|---|---|---|
| Control | T1 | T2 | T3 | |
| Flavor (60 points) | 58.42a | 56.72a | 55.30ab | 53.63b |
| Consistency and texture (30 points) | 26.11b | 28.84a | 29.06a | 27.15b |
| Appearance (10 points) | 8.25b | 9.16ab | 9.54a | 8.58b |
| Total (100 points) | 92.78a | 94.72a | 93.90a | 89.36b |
Where: a, b,c Means within the same row with different superscripts are significantly different (p £ 0.05). T1: Synbiotic yogurt enhanced with 1% ulvan polysaccharide, T2: Synbiotic yogurt enhanced with 2% ulvan polysaccharide, T3: Synbiotic yogurt enhanced with 4% ulvan polysaccharide.
Table 6: Sensory attributes of synbiotic yogurt enhanced with ulvan polysaccharide at fresh time.
The given scores for sensory assessment of synbiotic yogurt are illustrated in Table 6. The scores presented that the enhancement of yogurt with ulvan polysaccharide was acceptable in all treatments more than control. The lowest percentages of enhancement (1-2%) by ulvan polysaccharide were more accepted than the highest addition of this polysaccharide (4%) in all tested properties. From the flavor scores, it can be seen that by increasing the percent of added polysaccharide the given score was decreased and only significant (p ≤ 0.05) in the highest used percentage of ulvan polysaccharide in synbiotic yogurt (T3). These results may be due to the very slight like salty flavor which appeared in the higher treated samples (T2 and T3).
The last property in the used sensory card was the appearance of synbiotic yogurt, and the obtained scores followed the previous trend of consistency and texture. The lowest score was recorded for 4% ulvan polysaccharide-enhanced treatment, and this may be due to the existent of more amount of whey in this yogurt than the other treatments. Also, this result was compatible with that previous result of syneresis rate (Figure 3).
From the sensory evaluation of all treatments, it can conclude that the addition of ulvan polysaccharides only by 1 and 2% introduced synbiotic yogurt with good sensory attributes; and the obtained scores for texture and appearance were in accordance with that obtained results of physical properties.
What was investigated in this study is an attempt to manufacture healthy synbiotic yogurt containing probiotic and prebiotic together. Through the addition of ulvan which is water-soluble sulfated polysaccharide that extracted from macroalga Ulva lactuca. This polysaccharide has been known its nutritional and biological benefits. The results showed that adding 1-2% of the ulvan polysaccharide introduced synbiotic yogurt with good chemical and physical properties, as well as stimulated the growth and activity of probiotic bacteria. The addition of high percentage gave opposite results in terms of flavor, delayed fermentation, and weak texture with syneresis also. Therefore, it is recommended to use ulvan polysaccharide as prebiotic in the manufacture of synbiotic yogurt by adding 1-2%; and the flavor can be improved by adding any natural flavor to the synbiotic yogurt.
Citation: Shalaby MS, Amin HH (2019) Potential Using of Ulvan Polysaccharide from Ulva lactuca as a Prebiotic on Synbiotic Yogurt Production. J Prob Health 7: 208. doi: 10.35248/2329-8901.19.7.208
Received: 12-Jan-2019 Accepted: 11-Feb-2019 Published: 18-Feb-2019 , DOI: 10.35248/2329-8901.19.7.208
Copyright: © 2019 Shalaby MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.