Lupus: Open Access
Open Access
ISSN: 2684-1630
ISSN: 2684-1630
Mini Review - (2020)Volume 5, Issue 3
Systemic lupus erythematosus (SLE) is caused by the production of antibodies against self-antigens. The onset and/or progression of SLE appears to be associated with various viral infections. However, the underlying mechanisms of the association are not well understood due to the lack of suitable infectious experimental models. Recently, we demonstrated that a wide range of B cells are permissive to Theiler’s murine encephalomyelitis virus (TMEV), and TMEV-infected B cells are activated to up regulate co-stimulatory molecules for T cell stimulation. The initial activation of B cells requires the production of IFN-α/β.Subsequently produced IFN-α/β, IL-6, and IL-1 facilitate further B cell activation for antibody production and skewed development of Th17 type response. When SLE-prone BXSB and NZBWF1 mice were infected with TMEV and Coxsackie virus, the production of a wide range of autoantibodies in these autoimmune-prone mice was markedly accelerated. Several TLRs upon viral infections may participate in the B cell activation via NF-ҡB and critical cytokines such as IFN-α/β, IL-1, and IL-6. Therefore, TMEV infection in susceptible mice may offer an important tool to investigate the underlying mechanisms associated with B cell activation and consequent autoantibody production involved in SLE here.
Systemic lupus erythematosus, Neuropsychiatric lupus, Discoid lupus erythematosus, Neonatal lupus, Interferon, B cell lymphoma
SLE: Systematic lupus erythematosus; TMEV: Theiler’s murine encephalomyelitis virus; TLR: Toll-like receptor; DCs: Dendritic cells; Bcl: B cell lymphoma; IFN: Interferon; IL: Interleukin.
Systematic Lupus Erythematosus (SLE) is an autoimmune disease characterized by acute and chronic immune-mediated inflammation of various tissues of the body. Environmental risk factors may play a role in the pathogenesis of SLE, and its onset and/or progression is associated with various viral infections [1]. Certain viral nucleic acids aggravate autoimmunity through nucleic-acid-specific TLRs [2]. BXSB male mice containing duplicated TLR7 genes develop a lupus-like disease [3]. In addition, TLR3, recognizing double-stranded RNAs, appears to function as an inducer of a lupus-like disease [4]. Antibodies produced by the polyclonal activation of immature B cells are polyreactive and bind charged nucleic acid-protein complexes [5]. Despite the strong association between TLR-mediated signaling and the development of SLE, studies with animal models involving viral infections have been limited. The lack of investigations on the role of viral infections in the development and progression of SLE is partly due to the absence of a suitable infection model for SLE.
Infection of susceptible mice with Theiler's murine encephalomyelitis virus (TMEV) establishes viral persistence and induces a chronic progressive demyelinating disease [6]. We have previously observed that the production of several proinflammatory cytokines (IL-1β, IL-6, TNFα, and IFNα/β) is induced in various cell types of autoimmune-prone mice upon infection with TMEV [7]. The up regulation of TMEV-induced cytokine genes is mediated by mainly TLRs, resulting in the activation of NFҡB and IRFs [8]. TMEV-infected dendritic cells (DCs), but not peptide-loaded DCs, stimulate the development of Th17 cells from naïve virus-specific CD4+ T cells and the resulting Th17 cells play a critical pathogenic role in the development of TMEV-induced demyelinating disease [9]. Therefore, such viral infections lead to the activation of select cytokine genes which promote the development of pathogenic T cell type responses in various autoimmune diseases (Figure 1).
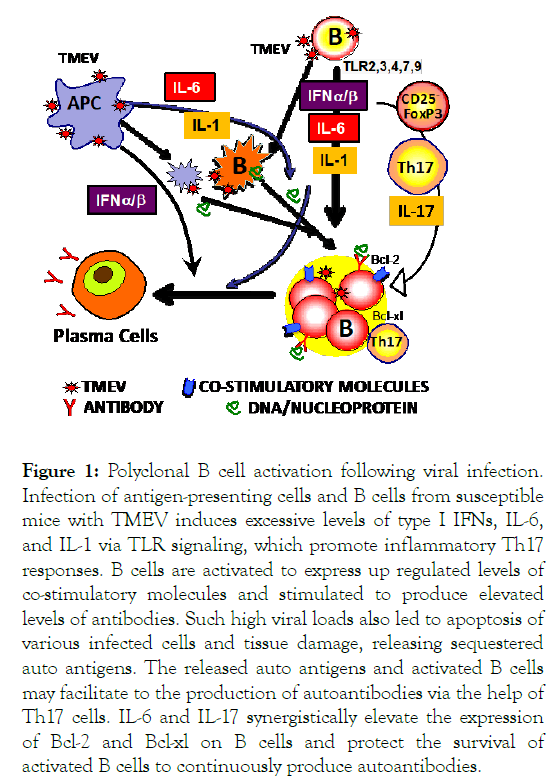
Figure 1: Polyclonal B cell activation following viral infection. Infection of antigen-presenting cells and B cells from susceptible mice with TMEV induces excessive levels of type I IFNs, IL-6, and IL-1 via TLR signaling, which promote inflammatory Th17 responses. B cells are activated to express up regulated levels of co-stimulatory molecules and stimulated to produce elevated levels of antibodies. Such high viral loads also led to apoptosis of various infected cells and tissue damage, releasing sequestered auto antigens. The released auto antigens and activated B cells may facilitate to the production of autoantibodies via the help of Th17 cells. IL-6 and IL-17 synergistically elevate the expression of Bcl-2 and Bcl-xl on B cells and protect the survival of activated B cells to continuously produce autoantibodies.
Recently, we demonstrated that primary B cells are permissive to TMEV infection similar to all other antigen-presenting cells [10]. Furthermore, the production of various autoantibodies was drastically accelerated in autoimmune-prone NZBWF1 and BXSB male mice following infection with TMEV or Coxsackie virus B3. A wide-range of B cell types were highly permissive (up to 40%) to TMEV. The infection of B cells with virus led to upregulated expression of MHC II and co-stimulatory molecules and the production of IFN-α, IL-6, and IL-1. IFN-α/β, IL-6, and IL-1 are known to activate B cells or B cell development [11,12], which are associated with the development of SLE [13]. IL-6 is a potent inducer of antibody production in conjunction with IL-21, which is produced by Th17 cells [14]. The skewed development of Th17 responses in the presence of excessive IL-6 and IL-1 was demonstrated with TMEV-infected DCs as well as B cells [9,10,15]. Th17 cells are also known to play an important role in the pathogenesis of SLE [16]. We have previously shown that IL-17 up regulates the expression of Bcl-2 and Bcl-xl prosurvival molecules in BM cells and renders the BM cells resistant to Fas/FasL-mediated apoptosis by T cells [9]. In addition, a combination of IL-6 and IL-17 synergistically inhibits cellular apoptosis [17]. Therefore, these cytokines likely promote the survival of B cells leading to persistent autoantibody production, which is critical for the pathogenesis of SLE (Figure 1).
In addition, as much as a two-fold higher proportion of FoxP3+CD4+ T cells in the CNS of virus-infected SJL mice displayed CD25lo [18], indicating that FoxP3+CD4+ Treg cells generated after TMEV infection may be functionally deficient [19]. Furthermore, activated TMEV induced FoxP3+CD4+ T cells did not inhibit the proliferative responses of CD4+ T cells reactive to the identical epitope and only suppressed the production of IFN-γ, not IL-17 [18]. Notably, high levels of CD25lo -FoxP3+CD4+ T cells were also observed in patients with SLE [20]. Because CD25lo FoxP3+ CD4+ T cells may undergo trans-differentiation into pathogenic Th17 cells [21], some or most of the CD25lo FoxP3+ CD4+ T cells may be converted into pathogenic Th17 cells in TMEV-infected mice.
Taken together, we believe that investigations with a viral infection model will provide valuable information regarding the development and progression of SLE. In particular, infection of TMEV and/or CoxB3 accelerates the production of polyclonal autoantibodies in autoimmune-prone mice and many immunological pathological parameters parallel to those of SLE. Some of the similarities include polyclonal B cell activation, involvement of identical cytokines, association with Th17, and defective FoxP3 Treg cells. Therefore, investigation of SLE pathogenesis with such a virus in depth may elucidate the underlying pathogenic mechanisms. Ultimately, these investigations may lead to the means to prevent or treat the development and/or progression of SLE.
Citation: Kim BS (2020) Potential Viral Model for SLE: Accelerated Polyclonal Autoantibody Production Following Theiler’s Virus Infection.Lupus: Open Access. 5:157
Received: 03-Nov-2020 Accepted: 19-Nov-2020 Published: 10-Nov-2020
Copyright: © 2020 Kim BS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.