Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Brief Report - (2023)Volume 12, Issue 1
Corrosion resistance behaviour of mild steel was evaluated in mineral acid using castor seeds as inhibitor. The potentiodynamic polarization measurement was carried out at room temperature for experimental data. The electrochemical experiment was carried at varying concentrations of the inhibitor. The extrapolation of the Tafel curves gave the corrosion rate and explained other parameters. The results obtained showed the susceptibility of mild steel without the inhibitor in mineral acids and its protective inhibition when different concentrations of castor seeds extract were used as inhibitor in the mineral acid environment.
Mild steel; Corrosion; Mineral acid; Castor seeds; Polarization; Inhibition.
Iron and its alloys are widely used for fabrication and engineering constructions. They are also known for thermal and electrical conductivity, easy fabrication and also exhibits good mechanical characteristics. The significant importance of iron and its alloys in utilization, in general and its protection in service in adverse corrosive environments has led to wide interest in research by scientist in regard of this metal and its alloys. Protection of iron and its alloys from corrosion has become a matter of great concern. Various chemicals have been used as inhibitors for this purpose but are threats to the nature and also are quite expensive.
Natural products can be considered as a good source for this purpose. The aqueous extracts from different parts of some plants such as fenugreek leaves [1], olive leaves [2], Datura metel [3], Eugenol derivatives [4], Rosemary oil [5, 6], Oil from Eucalyptus [7]. the essential oils from various plants such Lavender [8], Azadirachta indica [9], Osimum sanctum [10], Indian Gooseberry [11], Chamomile oil from Chamomilla recutita [12], Exyngium maritimum [13], Verbena [14,15] are having a reasonable corrosion inhibition on metals in aggressive media.
This work used 4N HCl as the test medium. Hydrochloric acid is a very versatile inorganic acid with wide industrial applications. This work aims at evaluating the corrosion resistance of mild steel in hydrochloric acid and its inhibition using castor seeds as inhibitor. It is anticipated that a good result will be obtained that could be of economic/technological benefit.
Preparation of specimens
The elemental analysis of the Mild Steel used in this work is shown in Table 1.
| Element | C | Si | Mn | P | Cr | Ni | Al | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Weight % | 0.076 | 0.026 | 0.192 | 0.012 | 0.05 | 0.05 | 0.023 | 0.135 | Bal. |
Note: 4N HCl; RT; castor seeds=1%
Table 1: Elemental composition of mild steel.
Mild steel panels of equal size (10 cm × 7.5 cm) were cut from single sheet of pickled cold rolled closed annealed mild steel (18 SWG) and used in all experiments. The specimens were polished to mirror finish with SiC emery paper, washed with ethanol for 10 min, then degreased with acetone for 1 min, followed by rinsing with ethanol and finally with deionized water. Finally, they were dried using hot air. For identification of specimens all were numbered and a suspension hole of about 2 mm diameter near upper edge was made. The corrosive acidic solution and other chemicals that were used in this work was prepared by diluting analytical grade quality reagents in deionized water. In the study, 4N solutions of acids were prepared.
The Castor seeds were dried, crushed, and powdered. This powder thus obtained was used as inhibitor. 1 mg of it was added to 100 cc of acid and kept for 24 hours. This acid was used for the preparation of inhibited pickling paste. Pickling paste was applied over weighed rusted panels under different conditions. After the experiment, paste was removed by washing with saturated sodium bicarbonate solution. The panels were again washed with water and dried with hot air. The panels were finally weighed to get the amount of rust dissolved.
Corrosion current experiment
Corrosion current was measured using ammeters of ma range by making galvanic couples of mild steel and platinum. Mild steel and platinum couple was put in acid solution (inhibited and uninhibited), they were connected through ammeter to record the corrosion current flowing through couple. Corrosion current as a function of time was measured.
Potentiodynamic polarization experiments
The electrochemical studies were made using a three-electrode cell assembly at room temperature. The mild steel was the working electrode, platinum electrode was used as an auxiliary electrode, and Standard Calomel Electrode (SCE) was used as reference electrode. The working electrode was polished with different grades of emery papers, washed with water, and degreased with acetone. All electrochemical measurements were carried out using Potentio-stat/Galvano-stat. This experiment was repeated about three times to ensure reproducibility.
SEM depiction
The morphology and chemical composition of the uninhibited and inhibited samples was examined using SEM as shown in Figures.
Corrosion current as a function of time for mild steelplatinum couple
Mild steel was connected to platinum and both were placed in 4N HCl with and without 1.0% Castor seed. Results given in Table 2 and Figure 1 show that in uninhibited system, when steel was connected to platinum, the starting current was 96 ma. The current gradually decreased with time. In one hour, the corrosion current is reduced to 83 ma. In inhibited system, the starting current was 78 ma which gradually reduced to 50 ma.
| Time (min.) | Current (ma) | |
|---|---|---|
| Uninhibited | Inhibited | |
| 0 | 96 | 78 |
| 10 | 95 | 72 |
| 20 | 92 | 66 |
| 30 | 89 | 60 |
| 40 | 89 | 57 |
| 50 | 88 | 54 |
| 60 | 83 | 50 |
Table 2: Corrosion current in steel platinum couple placed in paste.
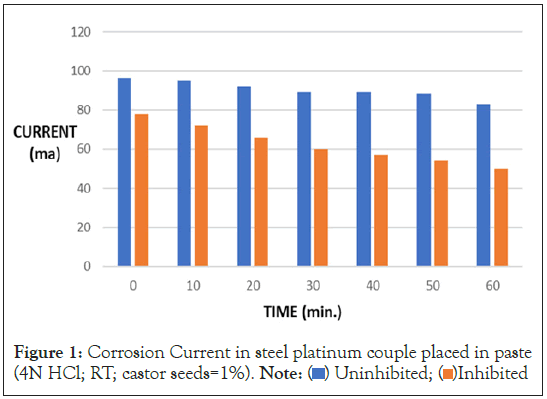
Figure 1: Corrosion Current in steel platinum couple placed in paste
(4N HCl; RT; castor seeds=1%). Note:  Uninhibited;
Uninhibited;  Inhibited
Inhibited
Polarization for mild steel
Anodic polarization: Table 3 and Figure 2 shows anodic polarization data for mild steel exposed to 4N HCl with and without 1.0% castor seeds. Results show that when current was raised from 3.3 × 10-3ma to 26.7 × 10-3ma, the potential increased from -656 mV to -616 mV for uninhibited system. In inhibited system, the potential varied from -652 mV to -530 mV.
| Current density (ma/cm2) | log current density | Potential uninhibited (mV vs. SCE) | Potential inhibited (mV vs. SCE) |
|---|---|---|---|
| 3.3 × 10-3 | -3.519 | -656 | -652 |
| 4.7 × 10-3 | -3.672 | -656 | -650 |
| 7.8 × 10-3 | -3.892 | -655 | -642 |
| 11.8 × 10-3 | -2.072 | -651 | -634 |
| 17.1 × 10-3 | -2.233 | -650 | -610 |
| 23.3 × 10-3 | -2.367 | -648 | -573 |
| 24.5 × 10-3 | -2.389 | -636 | -550 |
| 25.9 × 10-3 | -2.413 | -628 | -545 |
| 26.7 × 10-3 | -2.427 | -616 | -530 |
Note: 4N HCl; RT; castor seeds=1%
Table 3: Anodic polarization data for mild steel.
Figure 2: Anodic Polarization data for mild steel placed in paste (RT;
4N HCl; castor seed=1%). Note:  Uninhibited;
Uninhibited;  Inhibited.
Inhibited.
Cathodic polarization: Table 4 and Figure 3 show cathodic polarization data for mild steel exposed to 4N HCl with and without 1.0% castor seed. Results show a potential drop when current was raised from 1.3 × 10-3ma to 26.1 × 10-3ma for uninhibited system. For inhibited system, at minimum current density.
| Current density (ma/cm2) | log current density | Potential uninhibited (mVvs. SCE) | Potential inhibited (mV vs. SCE) |
|---|---|---|---|
| 1.3 × 10-3 | -3.1139 | -515 | -516 |
| 2.2 × 10-3 | -3.3054 | -515 | -517 |
| 3.9 × 10-3 | -3.5988 | -514 | -517 |
| 6.1 × 10-3 | -2.7789 | -517 | -521 |
| 7.6 × 10-3 | -2.8808 | -518 | -525 |
| 11.2 × 10-3 | -2.0492 | -523 | -526 |
| 14.6 × 10-3 | -2.1644 | -526 | -531 |
| 23.0 × 10-3 | -2.3617 | -530 | -536 |
| 26.1 × 10-3 | -2.4166 | -533 | -539 |
Note: 4N HCl; RT; castor seeds=1%
Figure 3: Cathodic Polarization data for mild steel (RT; 4N HCl;
castor seed=1%). Note:  Uninhibited;
Uninhibited;  Inhibited.
Inhibited.
Surface morphology
Figure 4 shows the SEM of uninhibited and inhibited samples. Figure 4 shows the SEM micrograph from the test sample after the experiment. The sample surface seemed slightly degraded but not significantly due to the protective action of inhibitor that had provided a protective film barrier on the surface. It must be noted that inhibitor consists of hetero atoms-Nitrogen and Oxygen. The presence of these electron donating atoms in the organic compound is crucial for efficient corrosion inhibition as they are known to have inhibitory effect, facilitating the adsorption of the inhibitors on the metal surface [16,17].
Figure 4: (i) SEM (Scanning Electron Microscopy) micrograph of mild steel in hydrochloric acid solution. (ii) SEM micrograph of mild steel in hydrochloric acid solution in the presence of inhibitor.
It can be concluded from this work that:
• Mild steel was susceptible to corrosion in acidic experimental conditions.
• The inhibitor gave relatively good and effective protection against corrosion. This was confirmed by both the gravimetric and electrochemical results. However, the inhibitor polarized anode to a considerable extent in comparison to cathode.
• The adsorption trend of the inhibitor to the substrate’s surface exhibited the Langmuir adsorption isotherm model. Factors which are known to influence the adsorption process include the distribution of charge in molecule, the nature and surface charge of metal, the chemical structures of organic compounds, and the type of aggressive media.
I am grateful to DBS (PG) College for letting me use their resources for the conduct of the experiments. I am also thankful to the editor and anonymous reviewers who helped with the current shape of the paper by their constructive and insightful comments and suggestions.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar]
Citation: Srivastava M (2023) Potentiodynamic Polarization and Gravimetric Gauging of Corrosion on Mild Steel in Acid Environment and its Protection. Organic Chem Curr Res. 12:300.
Received: 06-Jan-2023, Manuscript No. OCCR-23-21283; Editor assigned: 09-Jan-2023, Pre QC No. OCCR-23-21283 (PQ); Reviewed: 23-Jan-2023, QC No. OCCR-23-21283; Revised: 30-Jan-2023, Manuscript No. OCCR-23-21283 (R); Published: 06-Feb-2023 , DOI: 10.35841/2161-0401.23.12.300
Copyright: © 2023 Srivastava M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.