Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2020)Volume 5, Issue 2
Objective: Kawasaki Disease (KD) is an acute systemic vasculitis that can lead to ischemic heart disease resulting from coronary artery aneurysms. Intravenous Immunoglobulin (IVIG) resistance is a major risk factor for the development of Coronary Artery Lesions (CALs). Pulsed or non-pulsed steroids combination therapy as initial treatment for children with high risk KD was listed in the Japanese guideline in 2012. However, some patients with very severe KD do not improve even with the steroid combination therapy. The purpose of this study was to clarify the risk factors for severe KD patients who do not respond to the steroid combination therapy, and to confirm whether they can be predicted in the early stage. Methods: Forty hospitalized KD patients with high scores on all three Japanese prediction scoring systems and who received steroids with IVIG in initial treatment were prospectively recruited. IVIG+steroid-responder group (n=21) required no additional therapy and IVIG+steroid-resistance group (n=19) required additional therapy. Clinical data before and after the initial treatment were compared between each group. Results: Kobayashi and Egami scores before the initial treatment and the neutrophil count 2 days after the initial treatment were higher in IVIG+steroid-resistance group than that in IVIG+steroid-responder group independently. Kobayashi score ≥ 7, 2 points; Egami score ≥ 4, 1 point; and neutrophil count after initial treatment ≥ 11000/μL, 2 points. Patients who had three points or more were likely to need additional treatment (sensitivity 89%, specificity 76%). Conclusions: Predicting very severe KD who does not respond to the steroid combination therapy in the early stage is possible. Our new, original model may help to predict non-responders to the initial steroid combination therapy and prevent coronary artery aneurysms.
Pediatrics; Pediatrics scoring systems; Kawasaki disease; Non-responder; IVIG; Methylprednisolone; Prednisolone
Kawasaki disease (KD) is an acute systemic vasculitis in children and it is the most common cause of pediatric-acquired heart disease [1]. KD may lead to ischemic heart disease, myocardial infarction, and sudden death at a young age. Treatment with a high dose of intravenous immunoglobulin (IVIG) is the most effective therapy for the acute phase of KD and it significantly reduces the rate of coronary artery lesions (CALs). However, approximately 20% of patients with KD still have persistent or recrudescent fever after initial IVIG treatment [2]. IVIG resistance is a major risk factor for the development of CALs [3,4]. Some studies have reported scoring systems that predict initially IVIG-resistant patients at KD diagnosis, including the Kobayashi score [5], Egami score [6] and Sano score [7]. A randomized study have suggested that corticosteroids reduced fever and prevented progression of coronary artery complications resulting from KD [8,9]. These results suggest that a steroid regimen combined with IVIG could be an initial therapy for high-risk patients with KD. Based on these studies, the Scientific Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery published revised treatment guidelines for acute KD in 2012 [10]. Considering the steroids, such as 2 mg/kg/day prednisolone (PSL) or 30 mg/kg/ day intravenous methylprednisolone (IVMP), combination therapy for high risk patients is recommended in this guideline.
Therefore, since 2012, our team started to clarify high-risk patient using these existing systems and give them a steroid combination therapy as an aggressive initial treatment. However, there were some severe KD patients who resisted the steroid combination therapy and developed coronary dilatation around 7 days after the onset of fever. Such a patient needs more aggressive treatment strategy for preventing permanent CALs.
The present study aimed to assess prospectively the risk factors for severe KD patients who do not respond to the steroid combination therapy, and to confirm whether they can be predicted in the early stage.
Definition of KD severity
All KD patients were predicted to be IVIG responsive or resistant using three scoring systems (Kobayashi, Egami, and Sano) at the time of diagnosis. In our retrospective data (~2011), 14% (19 out of 136 cases) of the patients who had a low score on all three predictive scoring systems, 32% (31 out of 97 cases) of the patients who had high score on one or two predictive scoring systems, and 49% (19 out of 39 cases) of the patients who had high score on all three predictive scoring systems were the non-responders to IVIG (not published). Therefore, we defined patients who had low score on all three predictive scoring systems as low-risk patients, who had high score on one or two predictive scoring systems as moderate-risk patients and patients who had high score on all three predictive scoring systems as high-risk patients.
Study design and Subjects
We prospectively recruited the high-risk patients with acute KD who were admitted to our institute, between June 2012 and June 2017. Patients with structural heart disease, diagnosis after day 8 of illness (the first illness day was defined as the day of onset of fever) were excluded in the study. We diagnosed KD using the Japanese diagnostic guidelines for KD [11]. We gave all patients the same treatment according to the guidelines and observed the clinical data of before and 2 days after the beginning of initial treatment and clinical coarse.
Informed written consent was obtained from the parents after the nature of this study had been fully explained to them. The university Ethics Committee on Human Subjects at Fukuoka University Hospital and Fukuoka University Chikushi Hospital.
Treatment protocol
At For the initial treatment, IVMP 30 mg/kg, IVIG 2 g/kg and PSL 2 mg/kg/day in four divided doses were administered sequentially with heparin (10 U/kg/h, continuous infusion) and a histamine-2 receptor antagonist (1 mg/kg/day). In 2 or 3 days after starting initial treatment, if the patients were afebrile (<37.5°C) for more than 24 hours and C-reactive protein levels had decreased by half, the PSL dose was tapered to 1 mg/kg/day. The PSL dose was tapered every 2 or 3 days, and discontinued within 10 to 14 days after fever onset (duration of PSL administration ranged from 5–8 days) (Figure 1). If diagnosed on day 3 or earlier, we administered urinastatin 1 day before IVIG administration in anticipation of neutrophil elastase inhibitory effect in early onset [12]. For non-responders to initial therapy (persistent/recurrence of fever or the laboratory data >24 hours after initial treatment), we administered IVMP 30 mg/kg for 2 days and IVIG 2 g/kg as a second-line therapy, and cyclosporine as a third-line therapy. Infliximab was also administered for the third-line therapy as necessary, after December 2015 when it was covered by Japanese insurance.
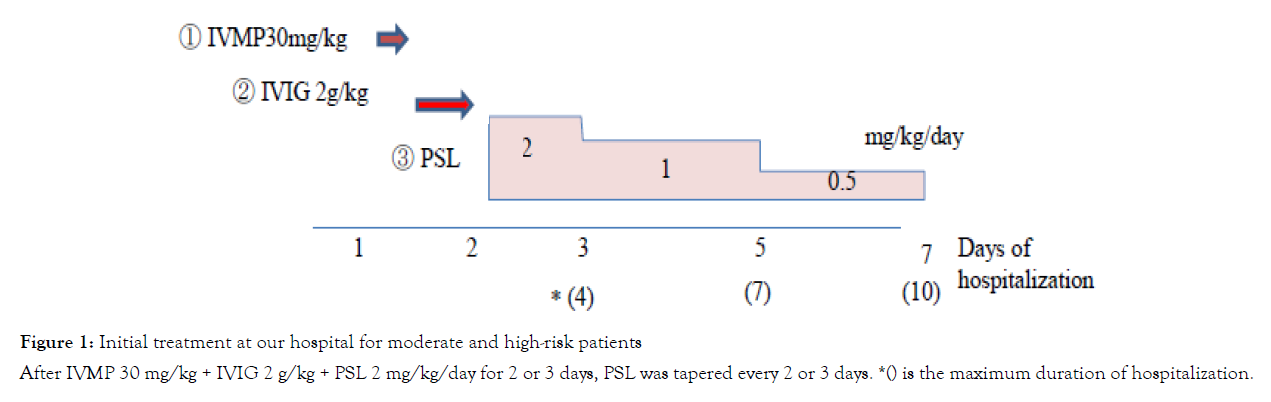
Figure 1: Initial treatment at our hospital for moderate and high-risk patients
After IVMP 30 mg/kg + IVIG 2 g/kg + PSL 2 mg/kg/day for 2 or 3 days, PSL was tapered every 2 or 3 days. *() is the maximum duration of hospitalization.
All patients with KD underwent complete 2-dimensional echocardiographic studies with color flow and spectral Doppler examinations (up to March 2016, Prosound α; Hitachi Aloka Medical systems, Tokyo, Japan, and April 2016 and thereafter, Vivid 7; GE Vingmed Ultrasound, Horten, Norway). The intraluminal diameters of coronary artery segments were measured from the inner edge to the inner edge. The presence of CALs was diagnosed based on the Z scores of the left main trunk coronary artery, the proximal left anterior descending coronary artery, and the right coronary artery [13]. We regarded > 2.0 SD as a CAL.
Statistical Analysis
Statistical analysis was performed using a t-test for continuous variables, the Mann–Whitney U test for nonparametric or extreme outlier continuous variables, and the chi-square test for percentages. We used Receiver Operating Characteristic (ROC) curve to determine cut off value. The correlation coefficient of the scoring systems for predicting non-response to IVIG was estimated using the list-wise method. We used multivariate logistic regression analysis to estimate the odds ratio (OR), and statistical tests were performed using the JMP 14 statistical software package (SAS Institute Inc., NC, USA). Only the characteristics that were associated with requiring additional therapy dropout with a p-value less than 0.20 in an unadjusted analysis were considered to be confounding factors and were included in the multivariable analysis. Differences were considered to be statistically significant at p<0.05.
Subject
There were 40 high-risk KD patients among all 235 KD patients. They were divided into two groups as follows. IVIG+steroidresponder group (n=21) those who did not required more than 2nd line therapy and IVIG+steroid-resistance group (n=19) those who required more than 2nd line therapy.
Patient characteristics
Patient characteristics are shown in Table 1. Mean age was 3 years in both groups. Mean day of diagnosis was around day 4 in both groups. There were no significant differences in the data between the groups.
| group (n=21) | IVIG+steroids-resistance group (n=19) | p | |
|---|---|---|---|
| Age (month) | 38.8 ± 18.7 | 45.5 ± 8.5 | NS |
| Male sex | 12 (57%) | 12 (58%) | NS |
| First visit (day) | 3.7 ± 0.5 | 3.3 ± 0.5 | NS |
| Diagnosis (day) | 4.0 ± 0.5 | 3.8 ± 0.0 | NS |
Table 1: Patient characteristics
Bivariate regression analysis: Bivariate regression analysis of the scoring systems before initial treatment and laboratory data after initial treatment are shown in Table 2. Before initial treatment, all three IVIG-resistant prediction scores in IVIG+steroidsresistance group were significantly higher compared with that in IVIG+steroids-responder group (Kobayashi score, 8 ± 1 vs. 6 ± 1, p<0.01; Egami score, 4 ± 0.5 vs. 3±0.5; p<0.05; Sano score, 3 ± 0.3 vs. 2 ± 0.5, p<0.01, respectively). After initial treatment, the white blood cell count, especially the neutrophil count, and C-reactive protein (CRP) in IVIG+steroids-resistance group remained at significantly higher levels compared with that in IVIG+steroidsresponder group (neutrophil count, 9000/μL ± 3794 vs. 7168/μL ± 2177, p<0.05; 5.1 ± 1.3 vs. 3.1 ± 1.6, p<0.05, respectively). AST and ALT in IVIG+steroids-resistance group also remained at higher levels compared with that in IVIG+steroids-responder group (AST; 52 ± 41 vs. 35 ± 12, p<0.05, ALT; 171 ± 50 vs. 79 ± 52, p<0.01, respectively).
| Before initial treatment | IVIG+steroids-responder group (n=21) | IVIG+steroids-resistance group (n=19) | P | P in logistic |
|---|---|---|---|---|
| Kobayashi score | 6 ± 1.0 | 8 ± 1.0 | 0.0004 | 0.0065 |
| Egami score | 3 ± 0.5 | 4 ± 0.5 | 0.0115 | 0.0073 |
| Sano score | 2 ± 0.5 | 3 ± 0.3 | 0.0095 | 0.7189 |
| After initial treatment | ||||
| White-cell count (/μL) | 10300 ± 4150 | 12300 ± 3200 | 0.0422 | 0.8158 |
| Neutrophils count (/μL) | 7168 ± 2177 | 9000 ± 3794 | 0.0385 | 0.1378 |
| Lymphocytes count (/μL) | 3353 ± 710 | 1630 ± 754 | 0.3253 | |
| Neutrophils/Lymphocytes | 7.2 ± 3.3 | 3.8 ± 4.6 | 0.1162 | 0.2532 |
| CRP (mg/dL) | 3.1 ± 1.6 | 5.1 ± 1.3 | 0.0101 | 0.2867 |
| AST (IU/L) | 35 ± 12 | 52 ± 41 | 0.0326 | 0.8532 |
| ALT (IU/L) | 79 ± 52 | 171 ± 50 | 0.0083 | 0.5794 |
| Total bilirubin (mg/dL) | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.0816 | 0.4323 |
| Albumin (mg/dL) | 2.9 ± 0.3 | 2.9 ± 0.2 | 0.5521 | |
| Sodium (mmol/L) | 138 ± 2 | 137 ± 2 | 0.3556 | |
Note: Data are presented as the median (interquartile range, IQR).
Table 2: Bivariate and multivariate logistic regression analysis before and after initial treatment
Multivariate logistic regression analysis: Characteristics that had a p-value lower than 0.20 in bivariate analysis were considered to be confounding factors and were included in the multivariable analysis. The p-value in the multivariate logistic regression analysis of only the Kobayashi score, Egami score, and the neutrophil count after the initial treatment had a p-value less than 0.20 (Table 2). We assigned these factors to the independent variable using multiple logistic regression.
The risk factors that predict non-responders to the steroid combination therapy
The risk factors were constructed to increase the usefulness of risk stratification using the independent variables; Kobayashi and Egami scores before initial treatment and neutrophil count after initial treatment (Table 3). Score points and cutoff points for each variable were as follows: Kobayashi score ≥ 7 (OR 17.0) was 2 points, Egami score ≥ 4 (OR 9.35) was 1 point, and the neutrophil count after initial treatment ≥ 12000/μL (OR 11.7) was 2 points. The median score for groups non-responders and responders were 3±1 and 1±1, respectively (p<0.01). The area under the ROC curve was 0.86. Observed rates of unresponsiveness to steroid combination therapy based on this new scoring model are shown in Figure 2. The patients who had scores 3 to 5, the requirement for additional therapy was 77%, whereas it was only 11.1% in the patients who had scores 0 to 2, and sensitivity and specificity were 89% and 76%, respectively.
| Risk Factor | OR | 95%CI | logOR | Points | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Kobayashi score | ≥ 7 points | 17 | 3 | 95.4 | 2.83 | 2 |
| Egami score | ≥ 4 points | 9.35 | 1.7 | 51 | 2.24 | 1 |
| Neutrophil count after initial treatment | ≥ 11000/μl | 11.7 | 1.3 | 106.8 | 2.46 | 2 |
Table 3: A model for predicting non-responders to the steroid combination therapy
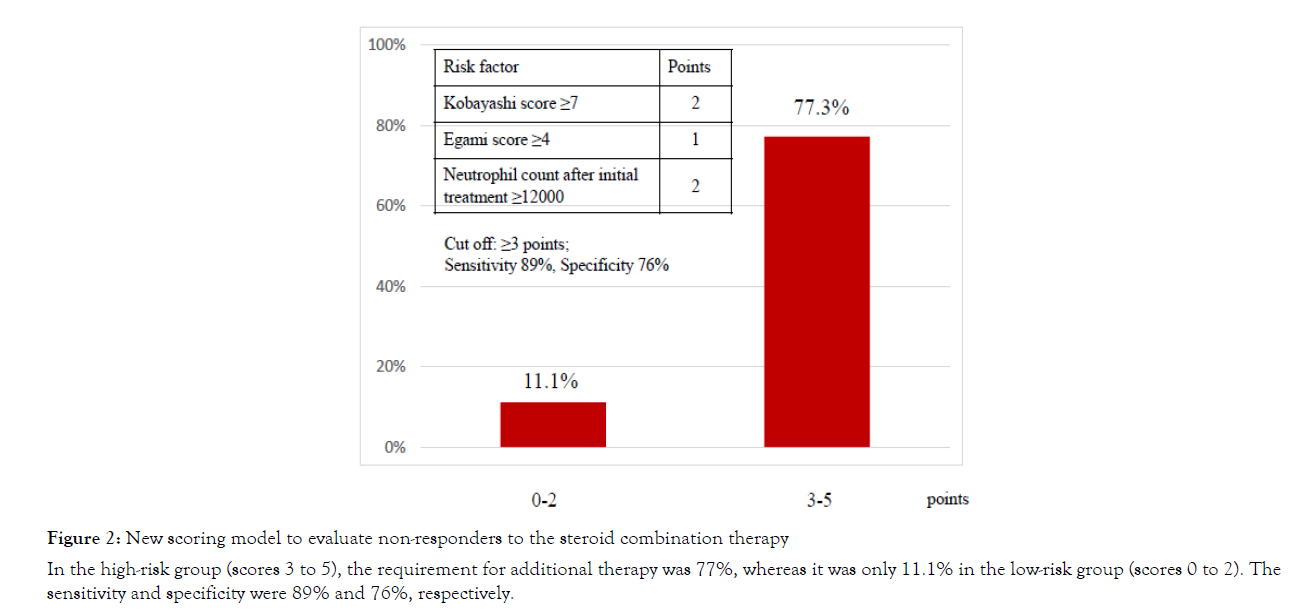
Figure 2: New scoring model to evaluate non-responders to the steroid combination therapy
In the high-risk group (scores 3 to 5), the requirement for additional therapy was 77%, whereas it was only 11.1% in the low-risk group (scores 0 to 2). The sensitivity and specificity were 89% and 76%, respectively.
Coronary artery lesions
There was no patient who had CAL before initial treatment. Prognosis of coronary arteries are shown in Figure 3. There were three patients who had enlarged coronary arteries. All of these patients had a high score using the new model and they required additional treatment. The details for these patients are shown in Table 4. Patient 1 who had 3 points using the new scoring model and 10332/μL neutrophils after the initial treatment had a CAL in the right coronary artery that was enlarged to +3.65 SD. This enlargement decreased within 2 months. Patient 2 who had 5 points using the new scoring model and 14767/μL neutrophils after initial treatment had a CAL in the left anterior descending artery that was enlarged to +5.21 SD. This enlargement decreased within 6 months. Patient 3 who had 5 points using the new scoring model and 24930/μL neutrophils after initial treatment had a CAL in the right coronary artery that was enlarged to +9.99 SD, in left anterior descending artery that was enlarged to +5.13 SD, and in left main coronary artery that was enlarged to +2.26 SD even with plasma exchange. The CAL in right coronary artery did not decrease and the patient has required anticoagulants to date.
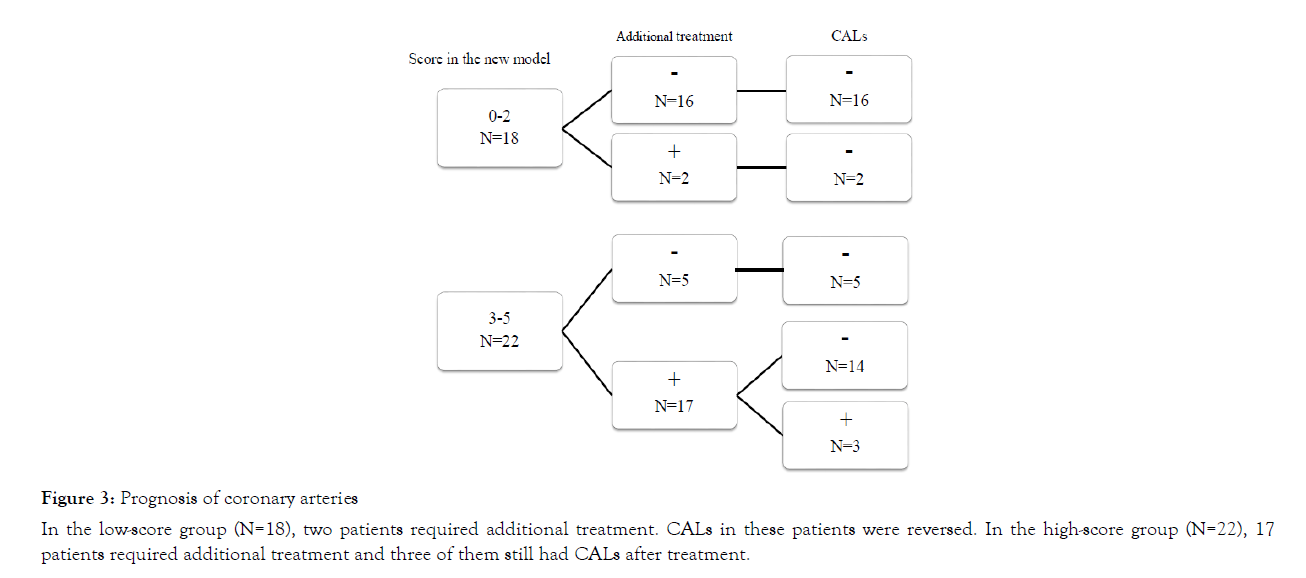
Figure 3: Prognosis of coronary arteries
In the low-score group (N=18), two patients required additional treatment. CALs in these patients were reversed. In the high-score group (N=22), 17 patients required additional treatment and three of them still had CALs after treatment.
| Patient | Age | Gender | Score of new models | Neutrophils count after initial treatment | Final | CAL | Timing of regression |
|---|---|---|---|---|---|---|---|
| additional treatment | +SD | ||||||
| 1 | 2 | Male | 3 | 10332/μL | CyA | 3.65 | < 2 months |
| 2 | 3 | Male | 5 | 14767/μL | IFX | 5.21 | < 6 months |
| 3 | 3 | Male | 5 | 24930/μL | PE | 9.99 | Not regressed |
Note: CyA - cyclosporin A; IFX - Infliximab; PE - Plasm
Table 4: Coronary artery lesions
Our findings showed that it is possible to predict non-responders to steroid combination therapy using the existing scoring systems to predict IVIG resistance before the initial treatment and the neutrophil count after initial treatment in the early stage of KD. There are some patients whose condition worsens and who had giant coronary artery aneurisms even with steroid combination therapy. Additionally, steroid administration reduces fevers even if inflammation is not completely controlled. This makes it difficult for physicians to judge whether the inflammation has decreased. We detected risk factors for predicting non-responders to the steroid combination therapy will be useful in assisting physicians to judge whether the inflammation will subside. We believe that fever recurrence at approximately 7 days after the onset is a risk factor for the development of CALs. By using our scoring systems and taking a more aggressive treatment strategy, we can treat very severe KD without delay and thereby prevent CALs complications.
There are three scoring systems that are used to predict nonresponse to IVIG in Japan. Although almost all physicians use only one out of the three scoring systems, we used all three scoring systems because this approach can stratify patients into three risk stages for IVIG resistance, including low, moderate, and high risk (Figure 1). However, it is the problem that these scoring systems fail in multiethnic populations. The Japanese scoring models used to predict patients who are at high risk in heterogeneous populations, such as in North America, has shown a low sensitivity of 33-42% [14,15]. However, KD patients show various degrees of severity. Therefore, development of an accurate worldwide predictive model, which may include some biomarkers or the patient’s genetic background, is required in the future.
We used our own protocol; sequential administer of IVMP 30 mg/kg, IVIG 2 g/kg and PSL 2 mg/kg/day with tapering, and discontinued it within 10 to 14 days after onset of fever. A previous report from 1979 suggested that there was a high incidence of CALs in patients with KD who received a prolonged course of PSL [16]. However, some more recent studies have reported that steroid combination therapy for initial treatment does not increase or reduces the incidence of CALs [8,17,18]. Another reports suggested that methylprednisolone plus IVIG combination therapy was effective for reducing inflammation reactions in the early stages [5,19]. We have used steroid combination therapy to treat KD patients since the Japanese guideline was revised in 2012. In our strategy using both methylprednisolone and prednisolone, administering PSL after treatment does not require prolonged steroid administration. The duration of steroid administration in our study was shorter than that in the RAISE study (5–8 days vs. more than 15 days, respectively) [9].
Some patients who received IVMP and IVIG on the initial day of treatment had hypothermia (35 degrees Celsius) as adverse effects. However, not administering aspirin for 2 days during the initial therapy prevented the effect. Although no prospective study has confirmed that aspirin reduces the incidence of CALs, aspirin has been the mainstay of treatment in KD patients for many decades [20]. Aspirin is not used only for its anti-inflammatory effects but also for its anti-platelet properties. In our assessment, if the antiplatelet agents such as aspirin are not administered for the first 2 days of initial therapy, no problems are expected because heparin is administered for its anti-coagulant effects. No other obvious adverse effect was occurred.
Our data from three patients who had CALs showed that the neutrophil count after the initial treatment was proportional to the CAL severity (Table 4). Yoshimura et al. reported that neutrophils in KD produced a significantly higher amount of nitric oxide (NO), which causes endothelial cell damage [21]. Our data reflected the impact of neutrophils on endothelial cell damage. Administration of steroids increased the neutrophil count, but it was not serious enough to interfere with evaluating severity in the patients.
In the present study, median age of both groups was 3 years-old. This may seem strange because it is known that the most common age of KD is one-year-old in Japan. Although not shown here, the median age of all 235 KD patient in this term was 2.2 years-old (low-risk: 1.4 years-old, moderate-risk: 2.0 years-old, high-risk: 3.7 years-old, respectively). This means that older age of patients tends to be more severe. Moreover, among the high-risk patients, the median age of IVIG+steroids-resistance group was older than that of IVIG+steroids-responder group (3.8 vs. 3.2 years-old) (Table 1).
This study has several limitations. The present study was a prospective study at a single institution, and the number of patients was small. It is necessary to conduct a multicenter, randomized study to confirm our results. Second, we did not evaluate in the moderate-risk KD patients. Some of these patients followed a severe course. The risk of CAL formation in the moderate highrisk KD patients need to be verified in future research. Finally, existing scoring systems for predicting IVIG resistance may not reflect the severity of KD in patients in Europe and the US. Steroid combination therapy is not recommended routinely in the American Heart Association (AHA) guidelines. Therefore, development of an accurate worldwide predictive model using biomarkers or the patient’s genetic background, would be require in the future.
This study demonstrated that it is possible to predict nonresponders to steroid combination therapy. Our predictive model may help with more aggressive treatment strategy to prevent CALs in very severe KD patients. Development of an accurate worldwide predictive model which eliminate KD associated CALs are required.
We thank Jodi Smith, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Dr. Yukako Yoshikane conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript.
Dr. Tatsuki Miyamoto and Dr. Junichi Hashimoto followed up the patients, collect and interpreted data, and reviewed and revised the manuscript.
Prof. Shinichi Hirose and Prof. Atsushi Ogawa supervised all of the following up of patients, collecting and interpreting data, and reviewed and revised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Citation: Yukako Yoshikane, Tatsuki Miyamoto, Junichi Hashimoto, Shinichi Hirose, Atsushi Ogawa (2020) Predicting Very Severe Kawasaki Disease Who Does Not Respond to Initial Steroid Combination Therapy: A Prospective Cohort Study. Clin Pedia OA 5:164 doi: 10.35248/2572-0775.20.5.164
Received: 18-Mar-2020 Accepted: 13-May-2020 Published: 20-May-2020 , DOI: 10.35248/2572-0775.20.5.164
Copyright: Yoshikane Y, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Competing interests: The authors have declared that no competing interests exist.