Journal of Theoretical & Computational Science
Open Access
ISSN: 2376-130X
ISSN: 2376-130X
Research Article - (2023)Volume 9, Issue 2
Dye lasers are commonly used in the optical investigation because their solutions in organic solvents deliver tunable, coherent emission. They exhibit intense fluorescent, owning to some specific spectroscopic characteristics. One drawback of the laser dyes is that it shows excessive Triplet-State Losses (TSLs) the lack of theoretical predictions of fluorescence rates, Inter System Crossing (ISC), and phosphorescence in laser dyes prompted us to report on the predicted rates of radiative and non-radiative transitions of some laser dyes. Structural engineering by some substituents that influence the simulated rates of coumarin laser dye derivatives for an efficient operation was investigated. The NH2 functional group renders the coumarin 120 more fluorescents with reduced TLS than the other investigated materials.
Fluorescence; Intersystem crossing; phosphorescence; Lifetime; Triplet-State loss
Coumarin is one of the very famous organic fluorescent materials. Coumarin derivatives have been widely applied in many fields, such as optical brightening agents, photobiological energy transfer processes, Light-Emitting Devices (LEDs) with the advantages of a sizeable conjugated system and rigid planar structure), laser dyes, medicine, and bio/ chemosensors [1-3].
Previous work noticed that we still lack theoretically predicted spectroscopic characteristics of efficient laser dyes [4]. This paper aims to simulate the missing excited-state dynamic parameters such as fluorescence, ISC, and phosphorescence rates of some coumarin laser dyes induced by different substituents, identified in Figure 1 and Table 1. The varying photophysical properties of coumarin and its various derivatives are sources of motivation that prompted us to study the architecture of the coumarin derivatives, which imparts changes in the electronic distribution within the molecular skeleton. And the effect of substituents on the excited state dynamic rates will be investigated by predicting the rates of fluorescence (kflu), intersystem crossing (kISC), and phosphorescence (kPho) in the laser dyes and related molecule (2) given in Table 1. The current study results will pave the way to highlight the molecular architecture that enables designing efficient laser dyes.
| Dye (ChemSpider ID) | X | Y | Z |
|---|---|---|---|
| Carbostyryl 7 (79637) | N | N | CH3 |
| 2-quinolinone (168395) | N | N | CF3 |
| Coumarin 120 (83285) | O | N | CH3 |
| Coumarin 151 (90930) | O | N | CF3 |
| Coumarin 4 (4444190) | O | O | CH3 |
Table1: Dyes studied, ChemSpider ID, and the natural charge on the oxygen atom in position 2 (see Figure 1).
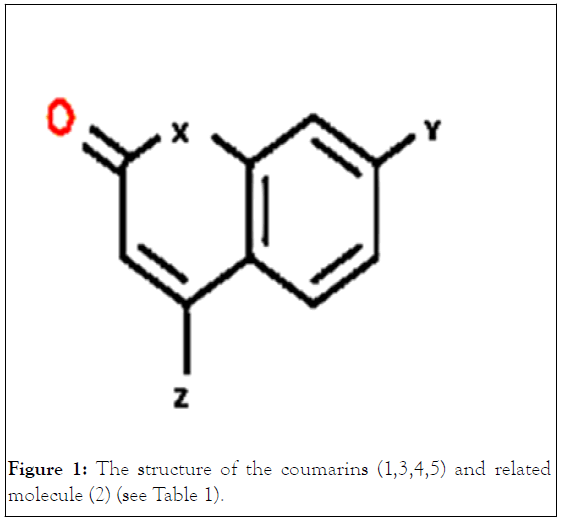
Figure 1:The structure of the coumarins (1,3,4,5) and related molecule (2) (see Table 1).
Computations
All calculations were carried out using the ORCA 4.2 (parallel) software package [5]. Structures were optimized using the B3LYP functional and the def2-TZVP (-F) basis set as recommended. The rates and spectra were calculated using the same methods recommended and detailed in the literature [6,7]. We considered the triplet spin-sublevels (1,0, or -1). For the molecules under investigation, we were predicted the kISC as the mean of the sum of the individual kISC (T1), kISC T2), and kISC (T3). We employed the well-known CPCM model of solvation [5] using ethanol as a solvent. An example of calculating the ISC rate for molecule 1 is given below:
!B3LYP DEF2-TZVP(-F) ESD(isc) TIGHTSCF GRID5 GRIDX5 RIJCOSX RI-SOMF(1X) CPCM(ETHANOL)
%TDDFT NROOTS 5
SROOT 1
TROOT 1
TROOTSSL 1
DOSOC TRUE
END
%MaxCore 1024
%scf ConvForced false END
%SCF MAXITER 300 END
%GEOM MAXITER 300 END
%ESD ISCISHESS "CARS7-ES.hess"
ISCFSHESS "CARS7-TS.hess"
USEJ TRUE
DOHT true
DELE 28643.09
END
%pal nprocs 35 end
| * xyz 0 1 O |
-3.111222000000 | 0.001372000000 | -2.059050000000 |
| N | -0.889634000000 | -0.889634000000 | -1.557789000000 |
| N | 3.886769000000 | -0.096090000000 | -0.770116000000 |
| C | -0.078363000000 | 0.036557000000 | 0.708754000000 |
| C | 0.181047000000 | 0.016771000000 | -0.691451000000 |
| C | -1.458950000000 | 0.029694000000 | 1.160873000000 |
| C | 1.038929000000 | 0.052927000000 | 1.571668000000 |
| C | 1.497780000000 | -0.000226000000 | -1.179933000000 |
| C | -2.469996000000 | 0.015302000000 | 0.243292000000 |
| C | 2.590167000000 | -0.005945000000 | -0.301008000000 |
| C | 2.338194000000 | 0.034378000000 | 1.094272000000 |
| C | -2.242567000000 | 0.009511000000 | -1.199275000000 |
| H | -0.720987000000 | -0.002120000000 | -2.559905000000 |
| H | 4.054014000000 | 0.173808000000 | -1.732364000000 |
| H | 4.622857000000 | 0.190972000000 | -0.136162000000 |
| H | 0.877614000000 | 0.081682000000 | 2.651303000000 |
| H | 3.179844000000 | 0.043742000000 | 1.791942000000 |
| H | 1.667446000000 | -0.024418000000 | -2.260897000000 |
| H | -3.518228000000 | 0.004283000000 | 0.548524000000 |
| C | -1.763582000000 | 0.031351000000 | 2.636938000000 |
| H | -1.301041000000 | -0.832830000000 | 3.143320000000 |
| H | -1.367091000000 | 0.936618000000 | 3.128295000000 |
| H | -2.847000000000 | -0.007917000000 | 2.817769000000 |
| * | |||
The predicted spectra of dye 3 (coumarin 120) are depicted in Figure 2 as an example. The theoretically simulated photophysical parameters are summarized in Table 2.
| Dye | kphos, s-1 | HT% | λphos, | kISC, | kflu, | λflu, | *kflu | *knr |
|---|---|---|---|---|---|---|---|---|
| (ms) | cm-1 | 109 s-1 | 109 s-1 | cm-1 | 109 s-1 | 109 s-1 | ||
| 1 | 0.30 (3333.3) | 91.1 | 14807 | 0.33 | 0.1 | 25126 | -- | -- |
| 2 | 0.62 (1612.9) | 98.5 | 14598 | 0.18 | 0.1 | 23529 | -- | -- |
| 4 | 5.40 (185.2) | 99.5 | 11854 | 0.09 | 0.15 | 22883 | 3.65 [10] | 54.3 [10] |
| 5 | 5.80 (172.4) | 99.5 | 16525 | 0.19 | 0.11 | 26810 | 0.22 [11] | 0.94 [11] |
| 3 | 294 (3.4) | 99.9 | 14116 | 0.14 | 0.17 | 24331 | 0.12 [12] | 1.24 [12] |
Table 2: Some predicted radiative (S1 S0, and T1 S0) and non-radiative (ISC) transitions of the dyes 1-5. Phosphorescence lifetime (τ) in ms is given between parentheses. The percent of the Hertzberg-Teller coupling (HT%) due to vibronic coupling is also p.
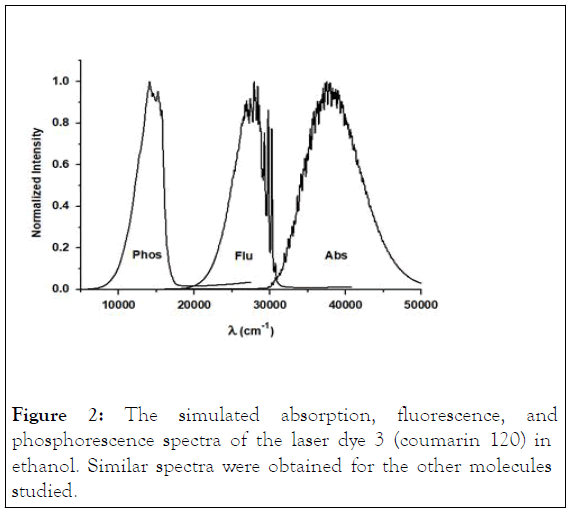
Figure 2: The simulated absorption, fluorescence, and phosphorescence spectra of the laser dye 3 (coumarin 120) in ethanol. Similar spectra were obtained for the other molecules studied.
Referring to Figure 1 and Table 1, architecturally, three important modified positions are observed in the coumarin skeleton for controlling the photophysical properties. One is the 1-position (X in Figure 1), and the second is 7-position (Y in Figure 1), and the third is the 4-position (Z in Figure 1). In this framework, the molecular engineering of these three positions by sensible substituent effect to understand the dominant deactivation channel and the laser efficiency of coumarin derivatives is addressable. Herein, two members of coumarin derivatives with O and N atoms substituted at the X-position were selected. For now, the rates of deactivation channels were successfully tuned by introducing substituents with variouselectronic properties at the Y-position (OH and NH2, Figure 1) and two-electron donor (CH3) electron acceptor (CF3) functional groups at the Z-position.
A glance at Figure 3 and 4 reveals the effect of substituents on the nonradiative
transition rate kISC and the radiative kflu.
Interestingly, a structural change in Y significantly influenced
fluorescence, ICS, and phosphorescence rates (see Table 2).
Substituting the OH group in dye 5 by the more electrondonating
NH2 group (dye 3) enhances kflu and kpho and
slightly depresses kICS. The triplet state lifetime is most
importantly shortened significantly, decreasing light loss by T  T absorption. In other words, shortening of a triplet-state
lifetime due to the replacement of the OH group by the more
electron-donating NH2 group prevents or limits excessive
Triplet-State Losses (TSLs.) and enhances laser dye efficiency.
Moreover, replacing the CH3 group in 3 with the more electronwithdrawing
CF3 group in 4 further decreases the kICS value
and renders dye 4 less efficient phosphorescent with a longer
triplet lifetime dye, enabling considerable TLS.
T absorption. In other words, shortening of a triplet-state
lifetime due to the replacement of the OH group by the more
electron-donating NH2 group prevents or limits excessive
Triplet-State Losses (TSLs.) and enhances laser dye efficiency.
Moreover, replacing the CH3 group in 3 with the more electronwithdrawing
CF3 group in 4 further decreases the kICS value
and renders dye 4 less efficient phosphorescent with a longer
triplet lifetime dye, enabling considerable TLS.
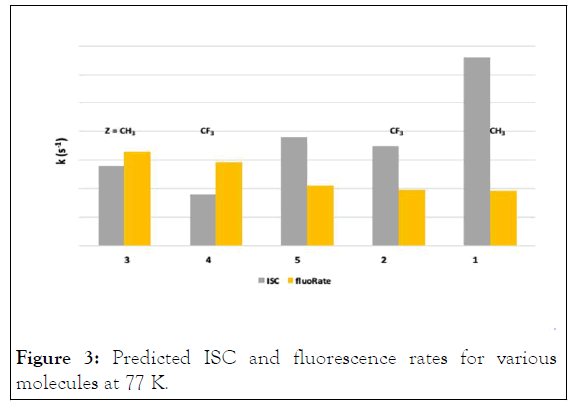
Figure 3: Predicted ISC and fluorescence rates for various molecules at 77 K.
Astonishingly, the N heteroatom in the X position, as in the carbostyryl 7 (dye 1), significantly depresses both kflu and kpho, noticeably enhances the kISC value relative to that of dye 3. Consequently, dye 1 should be a less efficient laser dye than dye 3. Molecule 2 behaves similarly to dye 1, indicating that its CF3 group is of minor influence in this case.
Noteworthy mentioning that the experimentally available photophysical data in the literature [4,8,9-12] match our theoretically predicted parameters (see Table 2). To the best of our knowledge, the triplet state characteristics of all the molecules studied are not reported in the literature [11, 12]. Previous experimental research [11, 12] rolled out the possibility of ISC based on the absence ofexperimental proofs. However, it was generally assumed that the ISC process is partially responsible for the fast nonradiative deexcitation channel for the fluorescent state of many dyes in nonpolar solvents.
This paper aimed to simulate the missing excited-state dynamic parameters such as fluorescence, ISC, and phosphorescence rates of some extensively reported coumarin laser dyes induced by different substituents. Structural substituents that influence the simulated spectroscopic rates of coumarin laser dye derivatives for an efficient operation have been highlighted.
We found that all the NH2-based dyes (3 and 4) show a much higher fluorescence rate with short-lived triplet-state than those of OH-(dye 5) or -N- heteroatom and NH2-based molecules (1 and 2). The results obtained pave the way to enable designing new efficient laser dyes.
There is no conflict of interest.
Citation: Abdel-Mottaleb MS (2023) Predictions of the Rate Constants of the Radiative Energy Dissipation and the Intersystem Crossing of Some Coumarin Laser Dyes. J Theor Comput Sci. 9:184.
Received: 02-Dec-2021, Manuscript No. JTCO-21-14102; Editor assigned: 03-Dec-2021, Pre QC No. JTCO-21-14102 (PQ); Reviewed: 10-Dec-2021, QC No. JTCO-21-14102; Revised: 23-Jun-2023, Manuscript No. JTCO-21-14102 (R); Published: 30-Jun-2023 , DOI: 10.35248/2376-130X.23.9.184
Copyright: © 2023 Abdel-Mottaleb MS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.