Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2024)Volume 14, Issue 1
Background: Traumatic Brain Injury (TBI) is one of the common preventable causes of mortality and disability among road traffic victims worldwide, especially in low-and middle-income countries, including Ethiopia.
Objective: To determine risk factors of mortality after traumatic brain injury due to road traffic crashes.
Methods: This study was done based on a prospective cohort of 242 severe brain-injured patients using cluster sampling in Addis Ababa city hospitals. The study was conducted from February 2018 to November 2019. Data were collected from brain-injured patients using a structured questionnaire, survival analysis was considered for statistical analysis. Ethical clearance was obtained from the Addis Ababa university, college of Health Sciences Institutional Review Board (IRB). Confidentiality of information was maintained.
Result: A total of 242 patients aged ≥ 18 years and diagnosed with severe traumatic brain injury, 73 (30.2%) died. The majority 186 (81%) of TBI patients were men. The median age of TBI patients was 29 years. The hazard ratio for patients with subnormal body temperature was 4.36 times that of normal temperature AHR: 4.36 (2.14-10.29). Patient with Glasgow Coma Scale (GCS) three to five was 5.61 times higher hazard ratio of death compared to GCS six to eight (CI: 3.1-10.24).
Conclusion: In conclusion, there was high risk of (30.2%) early mortality among TBI patients in Ethiopia. Being men, young and lower GCS were associated with higher mortality hazards. Hence, greater attention is needed for patients who experienced subnormal temperature and low Glasgow Coma Scale.
Road traffic crash brain injury; Brain trauma risk; Mortality after brain injury; Traumatic brain injury
CT: Computed Tomography; GCS: Glasgow Coma Scale; IRB: Institutional Review Board; TBI: Traumatic Brain Injury
Traumatic Brain Injury (TBI) constitutes a considerable portion of the global injury burden that leads to high levels of disability and mortality. Traumatic Brain Injury (TBI) is defined as an alteration in brain function or anatomic structure induced by mechanical force mainly due to a motor vehicle crash [1]. Mortality due to TBI is determined by two substantially different mechanisms/stages: Primary damage occurs when TBI happens due to initial impact, while secondary brain damage ensues through the development of mass lesions which are exacerbated by systemic insults as a consequence of intrinsic pathophysiologic mechanisms [2-4].
Studies estimated that 69 million people suffer from Traumatic Brain Injury (TBI) from all cases per year. The higher proportion of TBIs resulting from road traffic collisions occurred in both Africa and Southeast Asia (56%) and the lowest proportion in North America (25%) [5]. Low and Middle-Income Countries (LMICS) including Ethiopia have three times higher than TBI burden than high-income countries [6].
It is known about the large burden of TBI that constitute, high prevalence of long-term effects of the injury, loss to the work force, burden to healthcare systems and increased impact on issues such as family burden, social participation, country at large and health inequities represent [7]. Studies revealed an increased mortality rate following TBI [8-11]. TBI-related mortality is correlated with the severity of TBI at diagnosis [12,13].
Several factors contributed to TBI mortality, Glasgow coma scale score and presence of hematoma on CT as independently significant predictors of survival of TBI patient. Other studies show that significant predictors of time to death were males, younger age, being a driver, accident on interurban roads, time from injury to hospital arrival, hypoxia and systolic blood pressure on the admission of <90 mm Hg [14-19]. Significant morbidity and mortality occur when a patient experience both hypoxia and hypotension, even when it is merely a brief instance of each [20-22]. TBI patient mortality increases with the magnitude of hemodynamic instability and mainly the extent of the injury with bleeding [23-26].
In Ethiopia, Addis Ababa in particular, there were 28,361 crashes of which 4,433 of them harmed humans during 2019. But evidence on the epidemiology and outcome of severe traumatic brain injury due to road traffic crashes is limited. Therefore, the purpose of this study was to determine predictive factors of fatality after traumatic brain injury due to road traffic crashes and identify the extent of early mortality.
Study design, period and settings
We conducted an institutional base prospective cohort study, with follow-up of severe TBI patients from the time of injury in which admission within 24 hours of road traffic crash injury insured up to 30 days or earlier death. The research work was accomplished from February 2018 to November 2019 in randomly selected Addis Ababa city Tertiary hospitals, including Tikur Anbessa Specialized Hospital (TASH), Addis ababa Burn, Emergency and Trauma (AaBET) hospital and Minilic II hospital emergency departments. Addis Ababa city administration is the capital city of Ethiopia. Addis Ababa was founded by Emperor Minilik II and Empress Taitu in 1887. The history of the city ‘s road development also began from the inception of the city. Emperor Minilik II is also believed to be the first in importing two cars to Addis Ababa and to introduced car technology in the city for the first time in 1907 [27].
Source population
The source population was all charts of severe traumatic brain- injured patients due to vehicle crash that visited the desinated tertiary trauma care hospitals in Addis Ababa city.
Study population
Victims of road traffic crashes who were diagnosed with severe traumatic brain injury based on Computed Tomography (CT) scan finding recorded by examining medical doctors were the study population.
The study included every individual TBI patient with age greater or equal to 18 years old, having severe Traumatic Brain Injury (TBI) due to road traffic crash with measured intracranial lesion Abbreviated Injury Score (AIS) ≥ 4. Injury severity measured based on the base of the Computer Tomography (CT) scan findings recorded within the first 24 hours of admission. TBI patients with un-recordable vital signs, like pulse and blood pressure that indicates pre-hospital physiological status, during the pre-hospital time and were referred or transferred to other hospitals were excluded.
Sample size determination and sampling procedure
The study was initiated when an individual diagnosed with severe TBI and admitted in tertiary hospitals but have not experienced the death event at the time of ascertainment and prospectively followed to observe the death event. The participants were either experience the mortality event or censored on the 30th day offollow-up to exit the study.
Participants in this cohort were recruited based on brain injury severity status in which all those who fulfilled severe TBI diagnostic criteria and were admitted for it included. We ascertain risk factors such as age, sex, GCS, type of brain injury and TB severity status at the time of enrollment and then prospectively followed the individuals with the severe TBI to observe the death events of interest.
All tertiary care hospitals in Addis Ababa that provide trauma care services were identified. All hospitals with functional computed tomography scans were listed. The randomly selected three hospitals (Minilic II hospital, Tikur Anbessa hospital and AbaT hospital) that provide service to severe brain injury patients were recruited. The study subjects were recruited based on eligible criteria during the study period from the selected hospital’s emergency departments when the injured patients were admitted within 24 hours’ crash injury event.
Data collection instrument and techniques
Dependent variable: The response in this research was the “survival time/time for death”. It was defined as the number of days from the date of crash injury for which the patient arrived at the Emergency Department of hospitals assessed and followed up for 30 days for survival or death outcome. The survival data studied here were “right-censored”. Right censoring, were those cases like transfer-out, loss to follow-up and patients alive at the end of follow-up.
Independent variable:
• Socio-demographic characteristics: Age, sex, religion, marital status,
• Clinical factors: Traumatic brain injury type on the first day of crash occurrence, GCS, hypoxia, hypotension and hypothermia.
Operational definitions
Initial neuro-physiological variables: Glasgow Coma Scale (GCS) is a neurological scale aiming to provide a reliable, objective way of recording the conscious state of a person. Unconscious patients were defined as presenting a GCS <9. GCS assessment involves recording responsiveness in three domains: Eye-opening and motor and verbal responses. In a person who is fully conscious, alert and oriented, the glasgow coma scale will be E4 M6 V5 (15/15) and the reduction in the score is indicative of deterioration in the state of consciousness. The minimum score is E1 M1 V1 (3/15) who has no Eye-opening (E1), no Motor response (M1) and no Verbal response (V1) to any kind of stimuli. Glass coma scale was classified as severe (GCS 3-8), moderate (GCS 9-12) and mild (GCS 13-15) [28,29].
Prehospital arterial hypotension: It is defined as systolic blood pressure <90 mmHg measured at time point within 24 hours of crash injury.
Prehospital hypoxia: It is defined as body oxygenation with pulse oximeter oxygen saturation <90% measured at any time point within 24 hours of crash injury.
Prehospital hypothermia: It is defined as body temperature with a thermometer measurement ≤ 35.0°C measured at any time point within 24 hours of crash injury.
Fatality: It is defined as a severe traumatic injury patient’s death 30 days or earlier after the crash.
The data collection tool included a structured questionnaire responded to by severely injured patients or relatives. Besides, patient record data with measured traumatic brain injury severity and the vital sign was collected using a checklist adapted from another study [30]. The survival data for this study were obtained from selected brain-injured patients collected by emergency medical doctors and emergency Master of Science (MSc) nurses at emergency departments. The data collectors and supervisors with the principal investigator followed with the injured patients from the first day of crash injury assessment to 30 days for the patient’s survival or death.
Data quality assurance
Training was given to data collectors and supervisors about the objectives of the study and how to collect data.
The questionnaire was developed from previous studies, translated from English to Amharic (the national language) and back to English by language experts to ensure consistency. To ensure the quality of data, a pre-test of the data collection tool was done at Zewditu hospital. Supervisors and principal investigator facilitated the data collection process. The principal investigator worked with data collectors to assure the trustworthiness of the data and to minimize inter-observer bias. Every day, the collected data were checked for completeness and consistency using the data collection checklist. Double data entry was done to ensure accuracy.
Data processing and analysis
Data were coded and entered into the computer using STATA 14 statistical software package. Outliers were cleaned and validated data were prepared for analysis. In this study, survival analysis was employed. Descriptive analysis of survival data utilized nonparametric methods to compare the survival function of two or more groups. The Kaplan-Meier estimate (product-limit- estimate) of the survival function was employed. The Log-rank test was utilized to test whether observed differences in survival experience between/among groups were significant or not. The multivariable model used a semi-parametric regression model known as the Proportional Hazard Regression (PHR) model to estimate the hazard of death.
Result of the descriptive analysis
Over the 30 days of the follow up, 242 severe TBI cases were admitted to the three study hospitals. The cumulative incidence of death in this study was 73 (30.2%). Of those 59 (80.8%) were males. However, 169 (68.9%) were censored until the end of the study. Out of the total censored, 10 (5.8%) were transferred to other hospitals, 7 (4.1%) were lost at the end of follow-up and 153 (89.9%) were alive at the end of follow-up. The assessment was done by observing the patient during the hospital stay along with checking patient care records and cell phone calls.
Socio-demographic characteristics
The median age of TBI patients was 29 years. One hundred ninety-four (80.2%) TBI patients were men. The majority of TBI patients, accounting for 155 (64.1%) were pedestrians. One hundred fifty-four (63.6%) TBI occurred due to pedestrians injured by a fast-moving vehicle (Table 1). The highest number of participants from the 26 to 36 years’ age group (51.6%) died.
| Covariate | Category | Died | Outcome | ||
|---|---|---|---|---|---|
| Censored | Total | Present (%) censored | |||
| Sex | Female | 14 | 34 | 48 | 70.1 |
| Male | 59 | 135 | 194 | 69.6 | |
| Age | 18-29 | 34 | 89 | 123 | 72.4 |
| 30-39 | 15 | 45 | 60 | 75.0 | |
| 40-49 | 8 | 20 | 28 | 71.4 | |
| >=50 | 16 | 15 | 31 | 48.4 | |
| Type of road participant | Passenger | 29 | 45 | 74 | 60.8 |
| Driver | 5 | 8 | 13 | 61.5 | |
| Pedestrian | 37 | 116 | 155 | 74.8 | |
| Mechanism of crash | Hitting pedestrian by fast moving vehicle | 41 | 113 | 154 | 73.4 |
| Vehicle to vehicle crash | 18 | 28 | 46 | 60.9 | |
| Ejection | 8 | 18 | 26 | 69.2 | |
| Rolling | 6 | 10 | 16 | 62.5 | |
Table-1: Socio-demographic characteristics of TBI victims, Addis Ababa, Ethiopia, 2019 (n=242).
The Kaplan–Meier curve showed that estimated survival was 80% at 15 days of follow-up. The probability of survival decreased as the follow-up time increased. The highest rate of mortality occurred on the first day (Figure 1A).
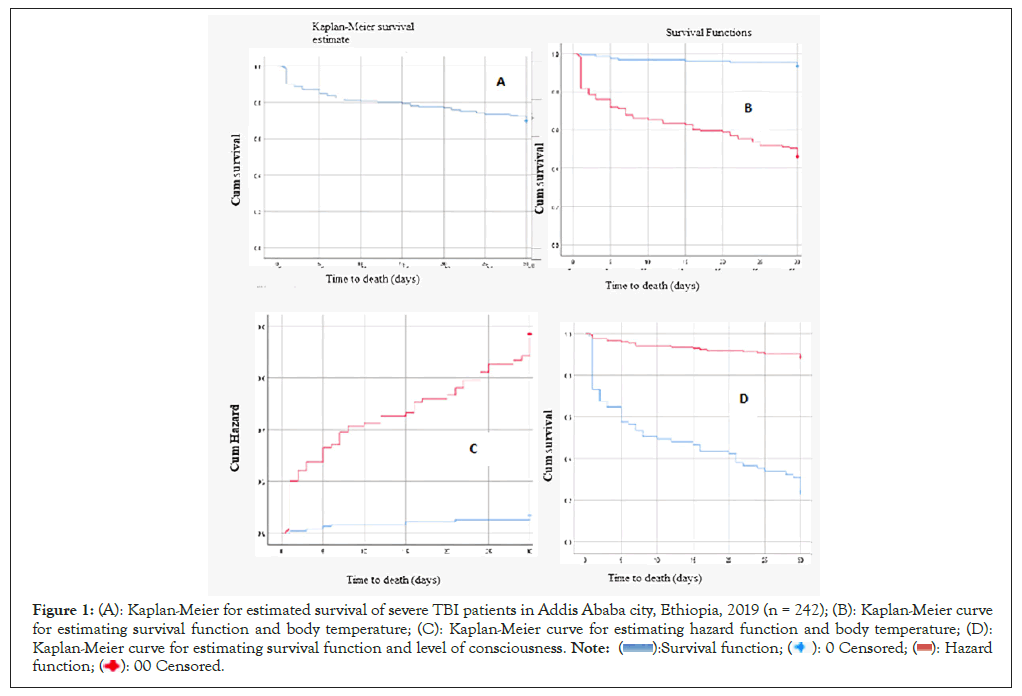
Figure 1: (A): Kaplan-Meier for estimated survival of severe TBI patients in Addis Ababa city, Ethiopia, 2019 (n = 242); (B): Kaplan-Meier curve
for estimating survival function and body temperature; (C): Kaplan-Meier curve for estimating hazard function and body temperature; (D):
Kaplan-Meier curve for estimating survival function and level of consciousness. 
TBI patients lived for an average of 24 days (95% CI: 22, 69- 25, 40 days). The incident rate was 0.365 per person-30 days. The incidence of death for age groups above fifty years old was 16 (52%).
Among 73 (30.2%) deaths, 65 (89%) were patients with subnormal body temperature. Fifty-four (74.9%) deaths were comatose patients. Ninety-four percent of TBI patients with normal body temperature survived for 20 days. A high proportion of TBI patients (40%) with subnormal temperatures died during the first 10 days. Overall Survival and hazard functions are shown (Figures 1B-1D).
Kaplan-Meier survival curve for estimating survival and hazard function (in days) of severe TBI patients in Addis Ababa city, Ethiopia, 2019 (n=242) (Figures 1A-1D).
Clinical characteristics
Eight (57%) of subarachnoid hemorrhage cases died. The hemorrhagic cerebral contusion cases, accounting for 19 (38%) also died. Regarding skull fracture, among 46 basal skull fracture cases, there were 22 deaths (47.8%). Out of 71 comatose patients, there were 54 deaths (76.1%). Out of 66 hypovolemic cases, there were 34 deaths (51. 5%). Out of 89 hypoxic cases, there were 46 deaths (51.7%). A summary of the data for each level of variables is provided (Table 2).
| Covariate | Category | Outcome | |||
|---|---|---|---|---|---|
| Died | Censored | Total | Present (%) censored | ||
| Type of injury | Subdural hematoma | 6 | 55 | 61 | 90 |
| Subarachnoid hemorrhage | 8 | 6 | 14 | 43 | |
| Intraventricular Hemorrhage | 20 | 33 | 53 | 62 | |
| Diffuse axonal injury | 17 | 30 | 47 | 64 | |
| Hemorrhagic cerebral contusion | 19 | 31 | 50 | 62 | |
| Epi-dural hematoma | 3 | 14 | 17 | 82 | |
| Skull fracture | Single bone fracture | 6 | 18 | 24 | 75 |
| Basal skull fracture | 22 | 24 | 46 | 52 | |
| Multiple bone fractures | 12 | 19 | 31 | 61 | |
| No fracture | 33 | 108 | 341 | 77 | |
| Body temperature | Normal | 8 | 113 | 121 | 93 |
| Subnormal | 65 | 56 | 121 | 46 | |
| GCS | GCS 3-5 | 54 | 17 | 71 | 24 |
| GCS 6-8 | 19 | 152 | 171 | 89 | |
| Systolic B/P | Systolic B/P >= 100 | 39 | 137 | 176 | 78 |
| Systolic B/P < 100 | 34 | 32 | 66 | 49 | |
| Oxygen saturation | Oxygen saturation >= 90 | 27 | 126 | 153 | 82 |
| Oxygen saturation < 90 | 46 | 43 | 89 | 48 | |
| TBI Injury | Isolated TBI Injury | 33 | 107 | 140 | 76 |
| Isolated TBI injury +other injuries | 40 | 62 | 102 | 61 | |
| Pre-hospital care |
Care given | 19 | 47 | 66 | 71 |
| No care given | 54 | 122 | 176 | 69 | |
Table 2: Clinical characteristics of TBI victims, Addis Ababa, Ethiopia, 2019 (n=242).
Table 3 shows results based on the log-rank test. The p-values show differences in survival experience between two or more levels of predictors. The predictors that manifested differences in levels of survival function are indicated as follows.
| Covariate | Degrees of freedom (df) | Chi-square (χ²) | Long-rank p-values |
|---|---|---|---|
| Sex of TBI patient | 1 | 0.01 | 0.91 |
| Ages of TBI patient | 3 | 9.43 | 0.24 |
| Marital status of TBI patient | 1 | 0.02 | 0.88 |
| Religion of TBI patient | 3 | 1 | 0.79 |
| Type of brain injury | 5 | 21.87 | <0.0006 |
| Isolated TBI injury + other site injuries | 1 | 7.2 | 0.007 |
| Systolic blood pressure measure | 1 | 22.08 | < 0.0001 |
| Oxygen saturation status | 1 | 36.78 | < 0.0001 |
| Body temperature | 1 | 66.04 | < 0.0001 |
| GCS | 1 | 127.27 | < 0.0001 |
| Pre-hospital care | 1 | 10.3 | 0.58 |
| Skull fracture involvement | 3 | 12.7 | 0.005 |
Note: TBI: Traumatic Brain Injury; GCS: Glasgow Coma Scale.
Table 3: Long-rank test p-values based on socio-demographic and clinical characteristics of TBI victims,Addis Ababa city, Ethiopia, 2019 (n=242).
Patients who had subdural hematoma injury lived for an average of ten days longer than those with subarachnoid hemorrhage injured patients. Patients, who experienced single lobe fractures lived for an average of six days longer than those exposed to a basal skull fracture. Patients with GCS measures ranging from three to five lived an average of 13 days lower than those patients with GCS measures ranging from six to eight. Normotensive patients lived for seven days longer than hypotensive patients. Hypoxemia patients lived for eight days longer than oxygen saturation patients. Isolated TBI lived for an average of four days longer than patients with TBI Injury plus other traumatic injuries.
Results of multivariate analysis
The cox model procedure that includes model selection, tests, diagnosis and fit confirmed no problems concerning the interaction of main effects and cofounding. Therefore, the results in Table 4 are based on the bi-variable and multi-variable analyses. It should be pointed out that variables with p-values below 0.05 were considered statistically significant. Based on bi-variable cox regression, the estimated Hazard Ratio (HRs) for those age groups greater or equal to 50 were associated with a poorer prognosis compared to the 19 to 29 age group (CHR: 2.18; CI: 2.21-3.96), the multi-variate model is not statistically significant. The result of the multivariate analysis revealed that the hazard of death for those patients with subnormal body temperature was 4.36 times than those with normal temperature (AHR: 4.36; CI: 2.14-10.29).
| Variables | Bi-variate analysis | Multi-variate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate | CHR (95% CI ) | P-value | Parameter estimate | AHR (95% CI) | P-value | |||||
| Age 18-29 | ||||||||||
| 30- 39 | 0.15 | 0.86 (0.47-1.58) | 0.62 | -0.199 | 0.44 (0.19-1.02) | 0.53 | ||||
| 40-49 | 0.03 | 1.03 (0.48-2.23) | 0.94 | 0.22 | 1.24 (0.56-2.74) | 0.59 | ||||
| >=50 | 0/78 | 2.18 (2.21-3.96) | 0.01 | 0.19 | 1.21 (0.65-2.24) | 0.55 | ||||
| Temperature | ||||||||||
| Normal | - | - | - | - | - | - | ||||
| Subnormal | 2.4 | 11.0 (5.67-22.97) | 0.001 | 1.55 | 1.64 (2.14-10.29) | 0.001 | ||||
| Systolic BP | ||||||||||
| Normal | - | - | - | - | - | - | ||||
| Subnormal | 1.04 | 2.83 (1.78-4.48) | 0.001 | 0.5 | 1.64 (1.00-2.69) | 0.05 | ||||
| Injury status | ||||||||||
| Isolated BI TBI + other | 0.61 | 1.84 (1.16-2.92) | 0.009 | 0.64 | 1.89 (1.17-3.05) | 0.009 | ||||
| Oxygenation | ||||||||||
| Normal | - | - | - | - | - | - | ||||
| Subnormal | 1.34 | 3.62 (2.37-6.16) | 0.001 | 0.34 | 1.4 (0.83-2.40) | 0.2 | ||||
| GCS | ||||||||||
| GCS 3-5 | - | - | - | - | - | - | ||||
| GCS 6-8 | 2.42 | 11.2 (6.59-19.03) | 0.001 | 1.73 | 5.61 (3.1-10.24) | 0.001 | ||||
Table 4: Estimated parameters of the bi-variable and multivariable cox regression analysis among TBI victims, Addis Ababa, Ethiopia, 2019 (n=242).
Based on multivariate cox regression, the estimated HRs of death for those who were hypotensive were 1.6 times higher than normotensive patients (AHR:1.6; CI: 1.00-2.69) and the multi- variate model was not statistically significant. Those patients who had traumatic brain injury plus other site injuries were 1.89 times more likely to die compared to those who had isolated traumatic brain injury (AHR 1.89; CI: 1.17-3.05).
Based on bi-variable cox regression, the estimated hazard of death for those patients who had hypoxia were 3.62 times higher than those of non-hypoxic (CHR: 3.62; CI: 2.37-6.16) and the multi- variate model was not statistically significant. The estimated fatality hazard for patients who experienced three to five GCSs was 5.61 times higher than for patients with six to eight GCS scores (AHR: 5.61; CI: 3.10 -10.24) (Table 4).
Test of proportional-hazards assumption
Testing the proportional hazard assumption is vital for the interpretation and use of fitted proportional hazard models. In this study, Goodness-of-Fit (GOF) particularly the Schoenfeld residuals proportional hazard assumption test for the individual covariates and global tests were used. The findings indicated that all variables included in the model satisfied the PH assumptions (p-value>0.05). For each predictor, there was no significant evidence of a poor fit. The global test does not have statistically significant evidence of poor fit (p-value: 0.67). Therefore; we do not reject the assumption of proportional hazards (Table 5).
| Covariate | Rho | Chi² | Df | p-value |
|---|---|---|---|---|
| Temperature | -0.045 | 0.10 | 1 | 0.67 |
| Systolic Blood Pressure | 0.072 | 0.41 | 1 | 0.52 |
| Injury status | 0.018 | 0.03 | 1 | 0.87 |
| Oxygenation | -0.19 | 3.27 | 1 | 0.07 |
| Glasgow Coma Scale | 0.15 | 2.08 | 1 | 0.15 |
| Age | ||||
| 18 -29 | - | - | - | - |
| 30- 39 | 0.16 | 0.79 | 1 | 0.18 |
| 40- 49 | 0.047 | 0.16 | 1 | 0.68 |
| >=50 | 0.085 | 0.56 | 1 | 0.45 |
| Global test | 5.78 | 8 | 0.67 | |
Table 5: Goodness-of-fit test assessing proportional hazards assumption among TBI victims, Addis Ababa, Ethiopia, 2019 (n=242).
This study finds out that patients with severe traumatic brain injury have a high mortality rate (30.2%), especially, males were victims. The rate of mortality reported in our study was higher than the study conducted in New York and Tunisia, which were 25% and 26.9% respectively and lower than the studies conducted in Uganda (33%), South Africa (39%). But our study involved only short-term mortality. Our study revealed that female Traumatic Brain Injury (TBI) patients had on average similar survival times to those of men. The majority of severely brain- injured patients, accounting for 80% were men which is similar to other studies conducted in Paris (84%), Tunisia (85.3%) and Uganda (80%). The possible explanation might be since male’s vehicle users are younger and energetic to engage in fast-moving vehicles that could lead to a crash. Our study showed that the mean age of severe traumatic brain injury was 36 years which is lower than the study conducted in the USA (40 years) and higher than the study conducted in Uganda (27 years) and similar to a study conducted in Paris (36 years). This difference might be explained by the fact that in our setting, younger victims were mostly exposed to crashes that lead to severe brain injury.
A previous study showed that primary injury has a wide range of TBI pathologies, such as diffuse axonal injury, focal contusions and space-occupying intra and extradural hematomas. That study was similar to our finding which revealed that 38% of deaths were due to hemorrhagic cerebral contusion. That might be due to not wearing a seat belt as a protective device.
Our study found out that the most fatal skull fracture was basal skull fracture and multiple skull fracture. Moreover, patients with TBI have often encountered other body site traumatic injuries. In this study, the traumatic brain injury patient who undergoes deep comma had the least survival. The estimated hazard ratio for those patients who experienced three to five Galvanic Vestibular Stimulation (GVS) score deep comma was more likely to die compared to comma patients. This is in agreement with other studies conducted in South Africa, Switzerland, Finland and Ethiopia. This indicates that a lower on-scene GCS is associated with higher mortality. This might be probably due to the worsening of primary impact. In the current study, hypotension resulted in a major fatality, accounting for 51.5% of deaths. Based on bi-variable cox regression, the estimated HRs for those who were hypotensive was 2.83 (CI: 1.78-4.48). This finding is similar to another study in New York. The majority of hypoxic severe traumatic patients (51.7%) died. Those patients with hypoxic had lived eight days shorter than those with non hypoxic. Based on bi-variable cox regression, the estimated hazard ratio for those with hypoxic were 3.6 times more likely to die compared to non-hypoxic (CI: 2.37-6.16). This finding was in agreement with previous findings that stated hypoxemia as a prehospital risk factor for the early fatality of severely brain-injured patients in Switzerland and Finland. This might suggest that hypoxia is a critical life threating situation of TBI patient.
The combination of hypotension and hypoxia occurring before arrival at the pre-hospital time is associated with a significant increase in death compared with either physiologic insult alone. This study was inconsistent with another study in America. This might be due to the primary impact of the brain tissue.
Methodological limitation of the study
This study may have some limitations: First, the data were collected where the crash injury victims have been in physical and psychological stress situations that can limit recalling the exact measure of scene and transport time values. Second, the study mainly focused on pre-hospital care of TBI, but prehospital data were not originally documented on the scene and during transportation for this study, but the data were verified by the supervisor and facilitator to reduce biases. Third, the study did not consider the previous status of the patient’s physiological status like hypovolemia and pathological status like comorbidity.
Fourth, collecting data at the time of hospital arrival may not be inclusive to collecting the overall situation of scene time and transport time characteristics of crash injured victims. Lastly, this study did not consider long-term mortality after 30 days of TBI and neurological deficit as a consequence of TBI, especially cognitive impairments that are not obvious during the study period.
In conclusion, there was high early mortality of TBI patients (30.2%) in Ethiopia. The lower GCS was associated with a higher mortality hazard, which may lead to a higher proportion of TBI patients with poor outcomes. Post-injury events, such as hypoxemia, hypotension and intracranial hypertension may also initiate the pathophysiological mechanisms of secondary brain injury. This demonstrates the need for early correction of the alarming situation in the pre-hospital setting to lower the risk of secondary brain injury. Trauma patient mortality increases with the magnitude of hemodynamic instability that requires appropriate skilled practice programs on pre-hospital TBI management. Further study on long-term outcomes of TBI including long-term mortality and disability to large extent should be considered.
We would like to express our deepest heartfelt thanks to the Addis Ababa university for allowing us to conduct this study. Our special thanks go to Tikur Anbessa, Minilic II and ABaT hospitals management committee staff for their support during the data collection process.
The authors extend their gratitude to the study subjects for their participation and willingness to be involved in the study.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Addis Ababa university, college of Health Sciences, Institutional Review Board (IRB) with protocol number: 036/17/SpH. Permission was obtained from selected Addis Ababa city Tertiary hospitals: Tikur Anbessa Specialized hospital, AaBET hospital and Minilic II hospital. Since some patients cannot read and write a crash victim and/or surrogate offered oral informed consent for free study participation according to the IRB-approved consent procedure. When patients were neurologically capable of giving informed consent, they were asked directly. If patients were not neurologically capable of giving informed consent themselves, their relatives were contacted and asked for consent. In the case of withdrawal, further, follow-up was discontinued. Complying with a patient’s request, the collected data would be removed from the database and destroyed. The confidentiality of information regarding patients involved in this study was maintained by keeping all patients’ records within the study site and avoiding identifying study participants by name on any documentation, report or publication resulting from data collected in this study.
All methods were performed with relevant guidelines and regulations.
Availability of data and materials
The data is presented along with the manuscript. All the data included in the manuscript can be accessed from the corresponding author Zuriyash Mengistu Assen upon request through an email address of zuriyashaau@yahoo.com.
Competing Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding
No specific fund is secured for this study.
Author’s contribution
ZM originated the idea, designed and developed a proposal participated in data collection, data organizing, statistical analysis and report writing.
AA participated in reviewing and editing-supervising. TA participated in reviewing and editing-supervising. All authors read and approved the final manuscript before submission.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mengistu Z, Ali A, Abegaz T (2024) Predictive Factors for Fatality after Traumatic Brain Injury among Road Traffic Crash Victims in Addis Ababa City, Ethiopia. Trans Med. 14:315.
Received: 13-Dec-2023, Manuscript No. TMCR-23-28484; Editor assigned: 15-Dec-2023, Pre QC No. TMCR-23-28484 (PQ); Reviewed: 29-Dec-2023, QC No. TMCR-23-28484; Revised: 08-Jan-2024, Manuscript No. TMCR-23-28484 (R); Published: 15-Jan-2024 , DOI: 10.35248/2161-1025.24.14.315
Copyright: © 2024 Mengistu Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.