Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2023)Volume 8, Issue 2
Aim: To explore the tolerance march in children and identify potential factors that affects the prognosis of Egg Allergy (EA).
Methods: Two hundred children (age range, 6 months-2.5 years) with Atopic Dermatitis (AD) were recruited from 2018 to 2019. EA was diagnosed based on medical history, Skin Prick Test (SPT), and the Oral Food Challenge (OFC) test. EA was diagnosed in 78 children; among these, 7 were allergic only to Egg Yolk (Only EYA), 20 to Egg White (Only EWA), and 51 to Whole Egg Allergy (WEA). Logistic regression analysis was used to identify risk factors for outcomes during the disease course. Receiver Operating Characteristic (ROC) curve analysis was performed to establish a predicting model.
Results: The Scoring Atopic Dermatitis score in the WEA group was more severe and persistent than that in the other groups. Forty-three cases of EA developed clinical tolerance (average age, 32.3 ± 8.7 months). The tolerance rate of EYA and EWA was 75.9% and 56.3%, respectively. The SPT wheal diameter at initial diagnosis (SPTdiag) was a risk factor for persistent EA. The SPT wheal diameter after 6 months (△SPT6mo) in the tolerant group was markedly decreased compared to that in the persistent EA group. Tolerance was higher when △EW-SPT6mo ≥ 39.5% or △EY-SPT6mo ≥ 27%.
Conclusion: The initial SPTdiag and SPT6mo values were significantly correlated with and can predict outcomes of EA.
Egg allergy; Atopic dermatitis; Outcome related factors
Egg Allergy (EA) is one of the most common food allergies that may occur very early in life [1, 2]. In 2011, Koplin, et al. [3] reported that the prevalence of food allergies in 1-year-old children is as high as 11%, including 8.9% prevalence of raw egg allergy. A birth cohort study involving 12,049 infants in 9 European countries found that the highest incidence of EA, as confirmed by the open food stimulation test, was 2.18% in the UK. In China, three cross-sectional studies of infants in the southeast city of Chongqing spanning 20 years documented an increase in the incidence of Immunoglobulin E (IgE)-mediated food allergies from 3.5% in 1999 to 7.7% in 2009, and 7.6% in 2019. Egg and milk are the most common food allergies in childhood in China [4, 5].
Immunoglobulin E (IgE)-mediated EA, egg allergy clinical manifestations were mainly skin symptoms, aggravates Atopic Dermatitis (AD) symptoms in infancy, with main manifestations of infantile eczema combined with digestive system and respiratory symptoms, which affect the growth, development, and quality life of the child [6]. However, eggs are also one of the main sources of dietary protein for infants and young children, which make EA with AD a common cause of skin complaints in this age-group. How to reduce blindly avoiding eggs in this age-group is also a challenge for pediatricians. Although natural tolerance occurs in 49.3% of infants at an average age of 3 years, the exact age bracket of tolerance and predictive factors in China remain to be determined due to paucity of research [7-9].
Mechanistically, five protein components in Egg White (EW) have been found to bind with human serum IgE to induce an allergic reaction, including Ovomucoid (OVM or Gal D 1), Ovalbumin (OVA or Gal D 2), Ovotransferrin (OVT or Gal D 3), lysozyme (Gal D 4), and ovomucin. In addition, Egg Yolk (EY) also contains antigens associated with allergies, namely yolk phosvitin, and yolk glycoprotein 42 (YGP42, Gal D 6) [10-11]. Because of the different antigenic components, clinical egg allergy is divided into three states EW allergy (EWA), EY Allergy (EYA), and Whole Egg Allergy (WEA) [12]. In this study, egg yolk and egg white Oral Food Challenge test (OFC) were respectively used for, identification of these three states. In addition, we explored the potential factors that predict clinical outcomes of EA. Our findings may help inform management strategies for EAs.
Patient’s selection
This study was approved by the Ethics Committee of the Ruijin Hospital of Shanghai Jiao Tong University School of Medicine, and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to their enrolment. From the pediatric allergy clinic of Ruijin Hospital, China, 200 children with AD aged 6 months to 2.5 years were included in the present study from 2018 to 2019. IgE-mediated EA was defined by OFC. Firstly, patients were screened using the criterion of positive Skin Prick Test (SPT) to EW or EY ≥ 3 mm and by maintaining a diet diary. Children whose AD symptoms (sleep status, itching, rash area and exudation) and digestive tract symptoms improved significantly after elimination of the whole egg from the diet were further evaluated by OFC .The antigen used in the SPT included fresh food obtained from EY, EW, cow's milk, wheat, fish, shrimp, peanut, and soybean.
Oral food challenges
The child was fed egg yolk (boiled at 100°C for 15 min) in stepwiseincreasing doses every 30 min, if no reaction was observed with the preceding dose. OFC was performed in the hospital by a trained pediatrician, and the subjects were observed for at least 2 hours after the last dose before going home. Parents were asked to report any symptoms that occurred in the subsequent 3 days. In case of occurrence of any reactions suspicious of EA, the children were to be brought to the hospital immediately for appropriate management in accordance with the EAACI guidelines [13]. Next, the egg white OFC was performed again after elimination of whole egg for 2 weeks. Any participant with an incomplete diagnostic process was considered a dropout. OFC was considered positive if the symptoms were improved after avoidance, and the symptoms reappeared after re-intake, and the diagnosis of EA was established. On the contrary, OFC was considered negative.
Groups
Children with AD who had negative results from both tests were recruited into the AD group (as control subjects). The Scoring Atopic Dermatitis (SCORAD) score and pruritus/sleep Visual Analogue Scale (VAS) score were used to evaluate the clinical baseline skin conditions. The 78 children with EA were divided into three subgroups as follows: 7 with only EYA (Only EYA), 20 with only EWA (Only EWA), and 51 with Whole Egg Allergy (WEA). At the end of follow-up, patients with EA were divided into tolerance group and persistent allergy group.
Follow-up
Children with AD were administered routine treatment, including topical skin moisturizing agents/glucocorticoids. Children diagnosed with egg ingredient allergy strictly avoided the corresponding ingredients, including oral intake and avoidance of contact in the family living environment. All patients were followed up at 3, 6, 12, and 18 months by recording the SCORAD score, itching/sleep VAS score, and respiratory symptoms (including nasal symptoms, chronic cough, wheezing, and asthma diagnosed by clinicians). SPT was concurrently conducted in the EA group. Tolerance was defined as negative OFC test: a wheal<3 mm and no allergic reaction after ingesting EY or EW for 3 consecutive days. OFC was also performed in the hospital by a trained pediatrician; positive OFC was considered indicative of persistent allergy.
Statistical analysis
SPSS v 25 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. The Mann-Whitney and Chi-squared tests were used to compare the characteristics of the tolerant and persistent groups. Using egg tolerance as the dependent variable, univariate and multivariate logistic regression analyses were conducted to identify risk factors, subsequently; the risk factors were used to plot the Receiver Operating Characteristic (ROC) curves. The optimal cutoff value for each risk factor was calculated using the Youden index to obtain the prediction model. Kaplan-Meierr survival analysis was used to determine whether the predictor could effectively predict tolerance development.
Among the 200 children with AD, 85(42.5%) were positive for egg SPT, of which 78 (39%) were diagnosed as EA based on OFC test. None of the subjects in the EA group were lost to follow-up or withdrew from the study, 56 had eaten eggs before diagnosis, 29 had simultaneous milk allergy. The duration of follow-up was 18 months in 43 children (55.13%) with egg tolerance and 35 with persistent EA. In addition, 48 children in the EA group presented with respiratory symptoms, which revealed that EA was associated with an increased risk chance of asthma (26.9 vs. 10.3%, P=0.039) when compared to that in the AD group.
Egg Allergy (EA)
In the present study, there was a 39% prevalence of EA in children with AD at a mean of 14.71 ± 7.9 months after an EA diagnosis. According to AD severity classification, 24 cases (30.8%) were mild, 41 (52.6%) were moderate, and 13 (16.7%) were severe. The median score of the itchy skin/sleep VAS self-rating scale (0-10) was 5 (4-7). The SCORAD score (P=0.012), severity grade (P=0.003), and itchy skin/ sleep VAS score (P=0.012) in the EA group were significantly higher than those in the AD group. There was no significant between-group difference with respect to the distribution range of EOS% between the two groups.
In the WEA group, 12 patients (23.5%) had severe AD, and 27 (52.9%) had moderate AD. Moreover, in the Only EWA group, milder AD (40 vs. 57%) and moderate AD (60 vs. 28%) were observed compared to that in the Only EWA group. In addition, we observed that the children in the WEA group had more skin damage (P=0.045) and sleep impairment (P=0.041) than those in the other groups.
Avoiding eggs and egg products significantly alleviated allergic symptoms in children with EA. The changes in SCORAD and VAS scores in the EA group were compared by Friedman-M method, and there were significant differences in different time periods (0, 3, 6, 12 and 18 months). In addition, higher median SCORAD and VAS scores were observed at different follow-up time periods in children in the WEA and Only EWA groups. Moreover, the change in the scores in children in the WEA and Only EWA groups was significantly slower than that in the other groups (P<0.001).
Egg tolerance and persistent EA
No significant differences were observed between the egg tolerance and persistent EA groups with respect to age, sex, premature birth, cesarean section, exclusive breastfeeding, only child, or respiratory diseases. The EA-persistent group comprised more children from a smoking environment than the tolerant group (P<0.05). There was no significant difference in the EA tolerance rate among children with different AD severity (i.e., mild, moderate, or severe).
Specifically, the average SPT wheal diameter at initial diagnosis (SPTdiag) in EY was 5 mm (3.25-6.875 mm) and in EW was 8.25 mm (6-10 mm) in the tolerance group. Additional follow-up results revealed that SPTdiag in the persistent group was 8 mm (6-9) in the EYA and 10.5 mm (9.25-15) in the EWA. Importantly, children in the persistent group had more persistent milk allergy (25.7 vs. 9.3%), nasal symptoms (54.3 vs. 30.2%), and asthma (40 vs. 16.3%) than those in the tolerant group (Table 1).
| Characteristics | Tolerant group (n=43) | Persistent group (n=35) |
|---|---|---|
| Age at diagnosis of egg allergy (mon) | 14.7 ± 7.8 | 14.6 ± 8.1 |
| Sex (man/male) (n) | 31/12 | 21/14 |
| Premature delivery (n) | 6 | 5 |
| Caesarean section (n) | 22 | 12 |
| Breast feeding (n) | 21 | 11 |
| Only child (n) | 31 | 27 |
| Smoking environment (n)* | 14 | 5 |
| History of allergy (n) | 27 | 26 |
| SCORAD score | 23 (13-32) | 22 (14.5-34) |
| Severity of AD | ||
| Mild (n) | 15 | 9 |
| Moderate (n) | 20 | 21 |
| Severe (n) | 8 | 5 |
| VAS score | 5 (4-6.5) | 5 (4-7) |
| EOS ≥ 5% (n) | 18 | 17 |
| Initial diagnosis of SPT wheal diameter (mm) | ||
| Egg yolk (mm)* | 5 (3.25-6.875) | 8 (6-9) |
| Egg white (mm)* | 8.25 (6-10) | 10.5 (9.25-15) |
| Persistent milk allergy (n)* | 4 | 9 |
| Multiple food allergies (n) | 18 | 16 |
| Symptoms of respiratory disease (n) | 26 | 22 |
| Rhinitis symptom (n)* | 13 | 19 |
| Chronic cough (n) | 11 | 10 |
| Wheezing (n) | 13 | 10 |
| Asthma (n)* | 7 | 14 |
Note: Data were expressed as median (interquartile range), *The P<0.05.
Table 1: Clinical characteristics of EA (Egg Allergy) in tolerance and persistent group.
Multivariate logistic analysis was carried out by using factors that were associated with P<0.1 in univariate logistic analysis. The results showed that EW-SPTdiag (P=0.031) and EY-SPTdiag (P=0.023) were independent risk factors for persistent EA, with odds ratio (or) values of 1.197 (95% confidence interval (CI): 1.016-1.411) and 1.470 (95%CI: 1.055-2.049), respectively (Table 2). In addition, logistic analyses were conducted with persistent EA and persistent milk allergy as independent variables and respiratory disease symptoms (nasal congestion, asthma) as dependent variables. We observed that persistent EA was associated with nasal symptoms (P=0.034) and asthma (P=0.022), and that children with persistent EA were more likely to develop nasal congestion and asthma.
| Regression analysis | B | S.E | Wald | df | Sig | Exp (B) | 95% C.I. for Exp (B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Smoking environment | 0.917 | 0.829 | 1.223 | 1 | 0.269 | 2.502 | 0.493 | 12.714 |
| EW-SPTdiag | 0.18 | 0.084 | 4.636 | 1 | 0.031 | 1.197 | 1.016 | 1.411 |
| EY-SPTdiag | 0.385 | 0.169 | 5.168 | 1 | 0.023 | 1.47 | 1.055 | 2.049 |
Table 2: Multivariable logistic regression analysis of the related factors of EA tolerance.
Tolerance to different egg ingredients
Egg tolerance was 86% in the Only EYA group, 60% in Only EWA group, and 49% in WEA group. The cumulative probability of allergic tolerance was calculated, there was a significant difference in the allergic tolerance rate among the three groups (P=0.03). Specifically, patients in the Only EYA group were the earliest and the fastest to develop tolerance, followed by those in the Only EWA group and those in the WEA group (Figure 1A).
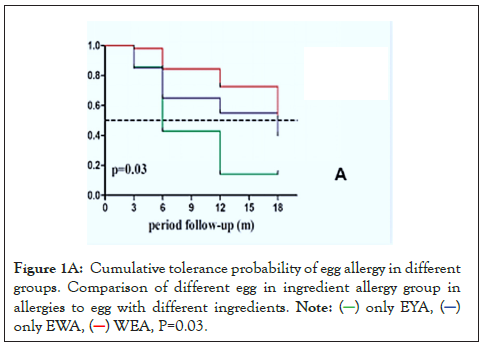
Figure 1A: Cumulative tolerance probability of egg allergy in different
groups. Comparison of different egg in ingredient allergy group in
allergies to egg with different ingredients. 
In the WEA group, 38 children were tolerant to EY and 28 children were tolerant to EW. The tolerance rates of EY and EW were also compared. We found that patients with EYA developed a tolerance faster than those with EWA (P=0.026) (Figure 1B).
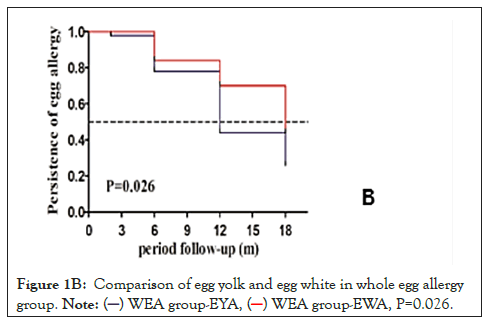
Figure 1B: Comparison of egg yolk and egg white in whole egg allergy
group. 
SPTdiag and △SPT6mo showed significant correlation with outcomes of EA
We next analyzed the relationship between the size of SPTdiag and the development of EA tolerance. Children with EA were divided into the EWA group (n=71) and EYA group (n=58). The larger was the SPTdiag for EW or EY; the lower was the probability of tolerance in the future. The SPTdiag for EY and EW (P<0.001) decreased at 0, 3, 6, 12, and 18 months of follow-up (Figures 2A and 2B). The median SPTdiag in the persistent allergy group was higher than that in the tolerated group (P<0.05).
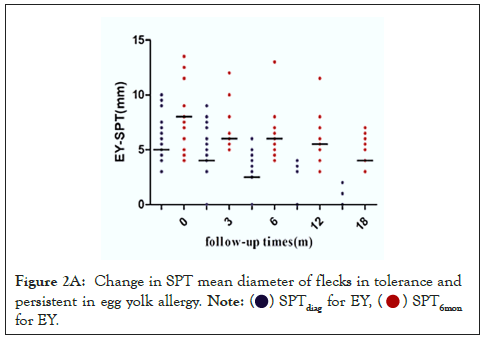
Figure 2A: Change in SPT mean diameter of flecks in tolerance and
persistent in egg yolk allergy.  for EY.
for EY.
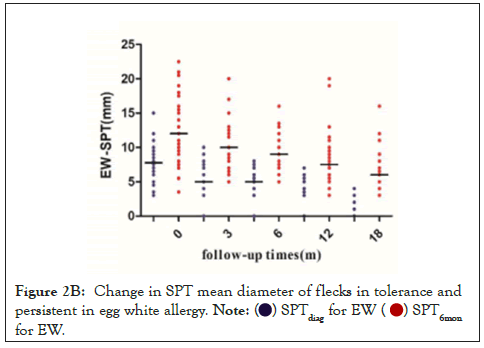
Figure 2B: Change in SPT mean diameter of flecks in tolerance and
persistent in egg white allergy.  for EW.
for EW.
To calibrate the SPTdiag conducted at irregular intervals, the reduction rate in the diameter was calculated at intervals of 6 months (ΔSPT6mo), using the following formula:
(ΔSPT6mo)%=[(V0: SPT-V2: SPT)/V0: SPT]*100, Where, V0: SPT is the first value of the mean perpendicular diameter of the SPTdiag, and V2: SPT is the value of the diameter of the SPTdiag in the second follow-up (6 months later).
Comparing the predictive power for tolerance acquisition in the EA group, ΔEY-SPT6mo Area under the Curve (AUC, 0.811; 95% CI, 0.692-0.929) was better than EY-SPTdiag (AUC, 0.787; 95%CI, 0.655- 0.919). The cutoff values were 7.75 mm for EY-SPTdiag and 27% for ΔEY-SPT6mo. The predictive power of using both ΔEY-SPT6mo and EY-SPTdiag (AUC, 0.750; 95%CI, 0.628-0.872) was similar compared to using EY-SPTdiag or ΔEY-SPT6mo alone (Table 3A). On comparing the predictive power in the EWA groups, ΔEW-SPT6mo (AUC, 0.836; 95%CI, 0.746-0.926) was better than that in the EW-SPTdiag (AUC, 0.722; 95%CI, 0.598-0.847). The optimal cutoff values were 10.25 mm for EW-SPTdiag and 39.5% for ΔEW-SPT6mo. The predictive power of using both ΔEW-SPT6mo and EW-SPTdiag (AUC, 0.798; 95%CI, 0.695-0.900) concurrently was similar to using EW-SPTdiag or ΔEWSPT6mo alone (Table 3B).
| Variables | Auc (95% CI) | Cut off value | P value |
|---|---|---|---|
| SPTdiag | 0.787 (0.655-0.919) | 7.75 mm | 0.01 |
| Δ SPT6mo | 0.811 (0.692-0.929) | 27% | <0.001 |
| SPTdiag+Δ SPT6mo | 0.750 (0.628-0.872) | 7.75+63.5% | 0.04 |
Table 3A: Prediction model of egg yolk allergy tolerance acquisition.
| Variables | Auc (95% CI) | Cut off value | P value |
|---|---|---|---|
| SPTdiag | 0.722 (0.598-0.847) | 10.25 mm | 0.02 |
| Δ SPT6mo | 0.836 (0.746-0.926) | 39.5% | <0.001 |
| SPTdiag+Δ SPT6mo | 0.798 (0.695-0.900) | 10.25+39.5% | <0.001 |
Table 3B: Prediction model of egg white allergy tolerance acquisition.
Patients were divided based on the cutoff ΔEY-SPT6mo value of 27% and ΔEW-SPT6mo value of 39.5% to perform additional analyses. Results showed that 42 patients had a ΔEY-SPT6mo ≥ 27%, and 38 patients (90.5%) developed EY tolerance. Of the children with a ΔEWSPT 6mo ≥ 39.5%, 30 (83.3%) developed egg tolerance. Kaplan-Meier curves showed higher incidence of development of EYA tolerance in the group with ΔEY-SPT6mo ≥ 27% (P<0.001) or ΔEW-SPT6mo ≥ 39.5% (P<0.001) (Figures 3A and 3B).
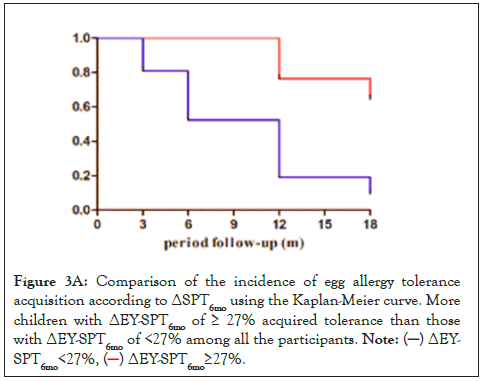
Figure 3A: Comparison of the incidence of egg allergy tolerance
acquisition according to ΔSPT6mo using the Kaplan-Meier curve. More
children with ΔEY-SPT6mo of ≥ 27% acquired tolerance than those
with ΔEY-SPT6mo of <27% among all the participants. 
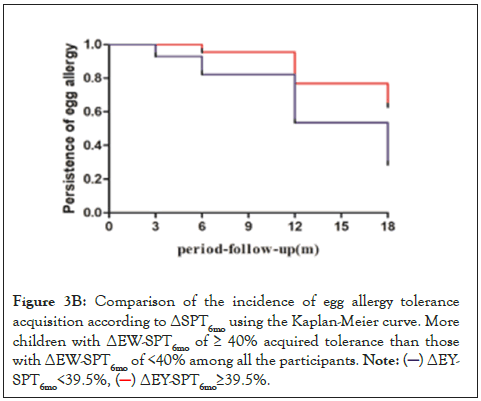
Figure 3B: Comparison of the incidence of egg allergy tolerance
acquisition according to ΔSPT6mo using the Kaplan-Meier curve. More
children with ΔEW-SPT6mo of ≥ 40% acquired tolerance than those
with ΔEW-SPT6mo of <40% among all the participants. 
Relationship between AD and EA
There is a certain degree of overlap in the allergy march. Results from a population-based cohort have shown that >50% of infants with AD have a FA, particularly in those with severe, persistent and early-onset eczema [14]. Infants with eczema were found 5.8 times more likely to develop egg allergy by the age months than infants without eczema. Studies have shown that early introduction is effective in preventing the development of a food allergy in specific groups of high-risk infants [15,16]. Mindlessly avoiding food can also increase the risk of malnutrition, and it is important to know if one is allergic to eggs. The mechanisms by which a food allergy develops after the onset of atopic dermatitis may involve damaged skin barriers and inflammatory synergism [17]. The reason why egg was found to be more closely related to AD is still unknown. According to a hypothesis, the interaction between the microbial proteins and the skin, and the presence of homologous proteins between microbes and egg allergen is related to the development of IgE sensitization and EA [18].
Consistent with this study, in the present study, the prevalence of EA in children with AD was 39%, and moderate and severe AD was strongly associated with EA. Children with EA had more serious itching/ sleep impairment, which had a negative effect on daily life. The skin symptoms improved significantly after avoiding the foods containing the allergens, although children with AD may have received routine treatment before entering this study. Some studies have mentioned that early onset AD (age: 2 years) is more easily relieved, and that moderate and severe AD are more likely to develop into persistent disease [19]. During the 18-month follow-up, the average age at development of tolerance was 32.3 ± 8.7 months, which suggested that the age of 2-3 years was the peak period for development of EA tolerance; however, the severity of AD had no effect on the development of EA tolerance. So far, avoiding eggs is the most important method by which to reduce EA.
Risk factors for the outcome of EA
In their 2014 study, Sicherer, et al. [20] reported a cohort of children with EA aged 3 to 15 months who were followed up for a median of 74 months. They found a significant correlation between low specific- IgE levels, skin symptoms, (such as urticaria or angioedema), and the EA outcome. On analyzing the factors that influence EA tolerance in the present study, we found no significant differences in sex, exclusive breastfeeding, multiple food allergies, premature birth, cesarean section, family history of allergy, or early ingestion of eggs between the EA tolerance and persistent allergy groups.
Eosinophil’s (EOSs) are the main immune cells mediating the delayed type of food allergic reaction, and eosinophil count in peripheral blood is a convenient marker of allergic status in clinical settings [21]. In the present study, we found that use of only the range of EOS% in infants and young children was of limited value in the diagnosis of rapid food allergy. In clinical settings, for infants with only a slight increase in the proportion of EOSs, a combination of medical history, serum specific IgE value, and SPT should be used to make a comprehensive judgment and a clear diagnosis before food avoidance.
Some studies have found a strong correlation between EA in infancy and subsequent sensitivity to inhaled allergens; in addition, persistent EA may be associated with the severity of asthma. We found that the prevalence of asthma was higher in children with EA, and that 49% of the cases with wheezing and chronic cough presented after 2 years. Persistent EA is a risk factor for nasal symptoms and asthma. In the allergy march, food allergy is a risk factor for asthma regardless of whether the suspected food is tolerated, and the asthma risk at the age of 4 years is 2-3 times higher than that for those with no history of food allergy [22]. Our results were consistent with the study by Rhodes, et al [23]. They investigated a cohort of infants born to 100 groups of parents with a history of allergic disease and found that SPT positivity and EA and milk allergy were independent risk factors for adult asthma. In the present study, we found that children with persistent milk allergies were more likely to have persistent EAs, which, is partly consistent with the study by Sicherer, which suggests that persistent milk allergy may be used as a predictor of EA tolerance.
In a retrospective study by Kose, et al. [24], at the end of 6 months of consuming BakFedhen’s Egg (BE), the scrambled egg tolerance showed a negative association with EW-specific IgE levels, EW SPT, and the EW Prick-To-Prick test (PTP). EW PTP was the most significant parameter. The study found that the size and rate of change in egg SPT wind mass is significantly related to the outcome of EA. SPTdiag was observed to be a risk factor for persisting EA. SPTdiag and △SPT6mo were significantly correlated with EA outcomes.
Tolerance of EA with different ingredients
Most of the children in the Only EWA group had moderate AD, while those in the Only EYA group had the least severe clinical symptoms that were relieved faster than that in the other groups. Children with EWA had more severe clinical symptoms and slower tolerance rates than children with EYA. Pérez-Rodríguez, et al. [25] found that EW protein affects the integrity of the intestinal epithelial cell–barrier function. EY plays an important adjuvant role in the EWA-induced Th2-type response, and EY may promote the sensitization of EW protein by activating innate immune cells. EW and EY have different antigenic components, and the tolerance rate for the different egg ingredients is also different.
During the 18-month follow-up, SPT decreased with time, which indicated that the size of the wheal is a predictor of egg tolerance. Specifically, the larger the diameter of the SPTdiag wheal, the less likely was the development of egg tolerance, and the more serious and longer lasting were the clinical symptoms. The cutoff value calculated from the ROC curve showed that EW-SPTdiag>10.25 mm and EY-SPTdiag>7.75 mm were associated with persistent EA. The decreasing rate of EWSPT wheal ≥ 40% and EY-SPT wheal ≥ 27% in 6 months suggested the development of egg tolerance. Regardless of whether it was EW or EY, the ability of △SPT6mo to predict the egg tolerance outcome was better than that of SPTdiag.
The diameter and rate of in the SPT (Skin Prick Test) wheal can well predict the development of tolerance to different egg ingredients. This serve as a useful method by which clinicians can guide the infants’ families to scientifically avoid and reintroduce allergic foods, which would finally improve and strengthen health management and decrease their financial burden. Of note, in practice, the results of SPT for fresh food are also affected by various factors, including the individual specificity and immune status of the subjects, the ability of the operator, and the type of immune response, all of which should be considered to be able to make a judgment according to the actual situation of the children.
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
[Crossref][Google scholar][PubMed].
Citation: Mo X, Lv J, Shao J (2023) Predictive Factors of Egg Allergy Clinical Outcomes in Infants and Young Children. Clin Pediatr. 8:234.
Received: 01-Feb-2023, Manuscript No. CPOA-22-20983; Editor assigned: 03-Feb-2023, Pre QC No. CPOA-22-20983 (PQ); Reviewed: 17-Feb-2023, QC No. CPOA-22-20983; Revised: 24-Feb-2023, Manuscript No. CPOA-22-20983 (R); Published: 06-Mar-2023 , DOI: 10.35248/2572-0775.23.8.234
Copyright: © 2023 Mo X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.