Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2015) Volume 0, Issue 0
Objectives: The study aims to evaluate the predictive value of a comprehensive index composed of simple biometric parameters in predicting apnea hypopnea index.
Subjects and Methods: Ninety-four adult patients with obstructive sleep apnea-hypopnea syndrome were retrospectively reviewed. Pharyngeal axial-computed tomography and polysomnography were applied before surgery. Minimal pharyngeal cross-sectional area, sleep-associated monitoring indices including apnea hypopnea index, and biometric characteristics including body weight were all recorded. Correlations between these parameters were analyzed using correlation and multiple linear regression analyses.
Results: Apnea hypopnea index was significantly correlated with minimal pharyngeal cross-sectional area (r=-0.390) and body weight (r=0.553). A notably higher correlation coefficient (r=0.681) was found between apnea hypopnea index and a new comprehensive index composed of minimal pharyngeal cross-sectional area and body weight data.
Conclusion: Apnea hypopnea index might be largely influenced by a combined effect of minimal pharyngeal cross-sectional area and body weight. The new comprehensive parameter combining minimal pharyngeal crosssectional area and body weight is an effective indicator for the evaluation of obstructive sleep apnea.
Keywords: Sleep apnea; Polysomnography; Body weight; Computed tomography
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a range of sleeping disorders and symptoms that includes apnea, hypopnea, and low blood oxygen saturation, snoring during sleep, and sleepiness during daytime. OSAHS is mainly caused by the frequent collapse of the narrow upper airway during sleep. Generally, the upper airway collapse occurs at the level of the uvula–soft palate complex and/or at the base of the tongue. The pathogenesis of OSAHS has not been fully defined because of its multifactorial trait. This process is affected by the interaction of many positive and negative factors, such as obesity [1,2], tongue shape [3], tongue volume/oral cavity volume ratio [4], muscular hypotonia of upper airway [5,6], and neck circumference [7,8].
Minimal pharyngeal cross-sectional area (MPCA) is an important risk factor of OSAHS [9,10]. Narrowing of the airway of oropharynx and/or hypopharynx is the principal cause of OSAHS. However, clinical tests conducted to predict the severity of OSAHS by using only MPCA obtained inconclusive results. Compared with patients with small MPCA, quite a few patients with large MPCA suffered from more severe clinical symptoms and showed higher apnea–hypopnea index (AHI). Apparently, other unknown factors, in combination with MPCA, increase the severity of OSAHS.
Thus, this retrospective study was conducted to determine the factors that affect AHI in combination with MPCA. Moreover, the study was implemented to elucidate the possible mechanism of the combined effect on OSAHS.
Subjects
Ninety-four adult patients with ages ranging from 20 to 69 years and with the typical symptoms of OSAHS, without any definite pulmonary or metabolic diseases, participated in the study. The OSAHS of the patients was diagnosed by nocturnal polysomnography. All the patients were recruited from the Otolaryngology–Head and Neck Department of General Hospital of Shenyang Military Area Command from January 2013 to December 2013. All the patients underwent surgeries that could relieve the symptoms of apnea and snoring. These surgeries included uvulopalatopharyngoplasty, hyoid suspension, and midline glossectomy.
Procedure
Patients were required to undergo physical examinations. Gender, age, height, and weight of each patient were recorded. Body mass index (BMI) was calculated by the formula: BMI=weight (kg)/height (m2).
The PolyWin polysomnography system (PSG, Respironics Inc., USA) was used for sleep monitoring. Data of experiment variables, such as electroencephalography, electrooculography, airflow at the nose and mouth, tracheal sound, and percutaneous arterial oxygen saturation, were recorded automatically. AHI, percentage of recording time with oxygen saturation below 90% (T90), apnea-hypopnea time/sleep time ratio (AT/ST), and lowest arterial O2 saturation (LaSO2) were obtained using the installed software. OSAHS grade classification was based on the criteria of the American Academy of Sleep Medicine [11]. Patients were categorized according to AHI into mild (5.00 to 14.99), moderate (15.00 to 29.99), and severe (=30.00) OSAHS.
High-resolution spiral computed tomography (CT) examinations (Lightspeed spiral CT scanner, General Electric Inc., USA) of the pharynx were performed to display and evaluate the morphology of the upper airway before surgery. The scan was conducted at the end of quiet inspiration. The patients were requested to stay in supine position without breathing or swallowing during the scans. For each patient, a series of consecutive axial section images with 2 mm width and 2 mm increments was collected. The computer window of the axial sections was set to a window width of 400 HU and a window level of 40 HU. The pharyngeal cross-sectional area on each axial section was measured thrice by the same operator. The mean value was used in the study. The data of cross-sectional area were calculated and provided by a computer program. Only the smallest measurement in each case was used for the study.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences version 17.0. The results were presented as mean ± standard deviation. The mean values were compared using an unpaired Student’s two-sided t-test. Multiple linear regression was employed to determine the relationship between AHI and biometric parameters, as well as new comprehensive indices. The quality of the regression was appraised by the coefficient of determination R2. Bivariate correlation was performed as well. Correlation coefficient was used to assess the association between two variables. Values of P<0.05 were considered statistically significant.
Sleep-associated monitoring indices
The AHI ranged from 6.4 times/h to 96.7 times/h. Three patients suffered from mild OSAHS. Twelve patients were diagnosed with moderate OSAHS. Fifteen patients with mild or moderate OSAHS were categorized as Group A (mild/moderate group). Group B (severe group) comprised 79 patients who were diagnosed by PSG with severe OSAHS.
The AHI, T90, AT/ST, and LaSO2 results are shown in Table 1.
| Index | Total n=94 |
Mild / Moderate n=15 |
Severe n=79 |
t value | P value |
|---|---|---|---|---|---|
| AHI(times/h) | 51.8 ± 21.4 | 20.5 ± 6.5 | 57.7 ± 7.7 | 7.99 | <0.001 |
| AT/ST | 0.38 ± 0.18 | 0.16 ± 0.07 | 0.43 ± 0.16 | 6.23 | <0.001 |
| T90 | 0.34 ± 0.24 | 0.14 ± 0.12 | 0.37 ± 0.24 | 3.67 | <0.001 |
| LaSO2 | 0.64 ± 0.14 | 0.69 ± 0.14 | 0.63 ± 0.14 | 1.76 | >0.05 |
Table 1: Polysomnography in 94 patients with OSAHS.
The AHI, AT/ST, and T90 results of the two groups showed highly significant differences (P<0.001). No significant difference was found in the LaSO2 results between the two groups (P>0.05).
3.2 Demographic and biometric characteristics.
The data of biometric characteristics and MPCA of the study subjects are recorded in Table 2.
| Variable | Total n=94 |
Mild / Moderate n=15 |
Severe n=79 |
t value | P value |
|---|---|---|---|---|---|
| Age(years) | 39.3 ± 10.3 | 44.9 ± 14.9 | 38.3 ± 9.0 | 1.662 | >0.05 |
| Height(cm) | 173.0 ± 5.7 | 169.6 ± 4.7 | 173.7 ± 5.7 | 2.607 | <0.05 |
| Weight(kg) | 84.9 ± 11.3 | 74.2 ± 8.6 | 86.9 ± 10.6 | 4.336 | <0.001 |
| BMI(kg/m2) | 28.3 ± 3.2 | 25.8 ± 2.4 | 28.8 ± 3.1 | 3.521 | <0.01 |
| MPCA(mm2) | 79.4 ± 41.7 | 105.3 ± 54.5 | 74.4 ± 37.3 | 2.720 | <0.01 |
Table 2: Biometric and phareyngeal characteristics of the study subjects.
No significant difference was found between the two groups in terms of age (P>0.05). Patients in Group B were significantly taller than patients in Group A (P<0.05). The differences in body weight and BMI between the two groups were highly significant (P<0.001).
The MPCA of all patients was defined to be at the retropalatal level or hypertrophic tonsil region. Sizes of the MPCA in the awake state ranged from 15.0 mm2 to 244.0 mm2. Global mean size was 79.4 ± 41.7 mm2. A significant difference was found in MPCA between the two groups (P<0.01).
3.3 Correlations between biometric parameters, new comprehensive indices, and AHI
The correlation coefficients between AHI and five biometric parameters are shown in Table 3. A low correlation was found with body height (r=0.259, P<0.05).
| Index | Age | Height | Weight | BMI | MPCA |  |
|---|---|---|---|---|---|---|
| AHI | -0.301** | 0.259* | 0.553 | 0.511 | -0.390 | 0.681 |
| AT/ST | -0.233* | 0.278** | 0.464 | 0.393 | -0.268** | 0.515 |
| T90 | N/C | N/C | N/C | 0.404 | -0.236* | 0.433 |
| LaSO2 | N/C | N/C | -0.298** | -0.348** | 0.233* | -0.299** |
Table 3: Correlations between parameters and sleep-associated monitoring indices (N/C: No correlation, *P< 0.05, **P< 0.01, The rest P< 0.001).
Correlations with weight (r=0.553, P<0.001; Figure 1A), BMI (r=0.511, P<0.001; Figure 1B), and MPCA (r=-0.390, P<0.001; Figure 1C) were highly significant.
Stepwise multiple linear regression analysis revealed that the most important influence factors were body weight and MPCA among the five independent variables (age, height, weight, BMI, and MPCA). The corresponding linear regression was defined by Equation A:
AHI=0.964 × W-0.165 × MPCA-16.945(R2=0.407).
We attempted to combine the two most important factors to formulate new comprehensive indices. Subsequently, we explored the correlation between AHI and the new indices. Body weight was a positive factor of AHI, whereas MPCA was a negative factor. Hence, the new comprehensive indices included seven ratios composed of weight as numerator and the nth root of MPCA as denominator. The ratios were W/MPCA, 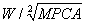 ,
, 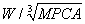 ,
,  ,
, 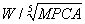 ,
, 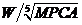 , had the strongest and most significant correlation (Figure 1D) with AHI compared with those of weight, MPCA, and the other six comprehensive indices. The corresponding linear regression was defined by Equation B: AHI=2.340 ×
, had the strongest and most significant correlation (Figure 1D) with AHI compared with those of weight, MPCA, and the other six comprehensive indices. The corresponding linear regression was defined by Equation B: AHI=2.340 × 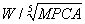 -33.637 (R2=0.464). 3.4 Correlations between new comprehensive indices and other sleep-associated monitoring indices.The indices AT/ST, T90, and LaSO2 also had correlations with the seven new comprehensive indices. Moreover,
-33.637 (R2=0.464). 3.4 Correlations between new comprehensive indices and other sleep-associated monitoring indices.The indices AT/ST, T90, and LaSO2 also had correlations with the seven new comprehensive indices. Moreover, 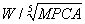 had the strongest correlation with AT/ST, T90, and LaSO2, compared with the other six comprehensive indices (data not shown).The correlations between all the sleep-associated monitoring indices and
had the strongest correlation with AT/ST, T90, and LaSO2, compared with the other six comprehensive indices (data not shown).The correlations between all the sleep-associated monitoring indices and 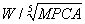 were stronger than those between sleep-associated monitoring indices and single parameters, except LaSO2 (Table 3).
were stronger than those between sleep-associated monitoring indices and single parameters, except LaSO2 (Table 3).
OSAHS is the result of the repetitive collapse of the upper airway during sleep. The participation of factors that could influence the upper airway collapsibility in the pathogenesis of OSAHS has been observed. Most previous studies focused on the effect of a single factor on sleep apnea.
However, accurate assessment of obstructive sleep apnea could not be obtained using only the data derived through a single parameter. Thus, we investigated the correlation between AHI and the combination of multiple factors to clarify the correlation and create a new method for assessment of AHI with simple data.
The pharynx, particularly the oropharynx and hypopharynx, is the region where most obstructive processes leading to apnea are found [12]. During the sleep process, the pharyngeal airway narrows because of structural abnormalities and reduced expansion forces of the pharyngeal dilator muscles [13].
The negative pressure inside the chest generated by the contraction of inspiratory muscles spreads to the narrow pharyngeal region. When the negative pressure exceeds the upper airway dilator muscle activity, the transmural pressure at the narrow region falls below a critical level.
Consequently, the upper airway soft tissue will be sucked inwards by the negative pressure, resulting in partial or full collapse of the upper airway and episodes of sleep apnea [6,14,15].
Thus, the stronger negative pressure power is produced in the chest, the more severe collapses of the upper airway will occur.
Obesity is the most common and well-recognized risk factor for OSAHS. A higher proportion of OSAHS patients are overweight [16,17]. BMI is a good indicator of obesity. In this study, BMI revealed a correlation with AHI (r=0.511). Notably, we also found a positive correlation between body weight and AHI (r=0.553). The mean body weight of Group B was larger than that of Group A.
However, body weight is a simple biometric parameter and could not profile obesity. Thus, one question remained unanswered: Why did body weight have a significant positive correlation with AHI?
Compared with body weight and MPCA, the new comprehensive index  significantly improved the correlation with AHI, AT/ST, T90, and LaSO2. The result brought about the question: Why did the new comprehensive index have a stronger correlation than any one of the two single factors?
significantly improved the correlation with AHI, AT/ST, T90, and LaSO2. The result brought about the question: Why did the new comprehensive index have a stronger correlation than any one of the two single factors?
We attempted to provide the answers to the two questions above. Tidal volume (TV) has an extremely strong positive correlation with body weight. Clinically, mechanical ventilation is performed on patients with suitable TV according to the measured or ideal body weights [18-20].
Normally, patients with higher weights have larger TV and larger thoracic volume change. TV, instead of weight, might play an important role in OSAHS occurrence with MPCA. In other words, the upper airway collapse might be affected by the joint effect of TV and MPCA during sleep.
To demonstrate the possible mechanism, we assumed two possible scenarios. First, two OSAHS patients have the same MPCA, but markedly different body weights and TVs.
During inspiration in sleep, stronger negative pressure is generated in the chest of the patient with more weight by larger thoracic volume change compared with that of the patient with less weight and smaller TV. Apparently, the upper airway wall of the patient with larger TV is more prone to collapse (Figure 2).
Second, two OSAHS patients have same body weight and TV, but markedly different MPCAs. During inspiration in sleep the same negative pressure is generated in the chests of both patients by the same thoracic volume change. However, the patient with smaller MPCA is more prone to oropharyngeal collapse and severe obstruction of the upper airway (Figure 3).
The analysis of the two scenarios shows that the oropharyngeal collapse might be influenced by both MPCA and the negative pressure in the chest. To a large extent, AHI can be determined by the joint effect of the two factors. Negative pressure in the chest is mainly determined by thoracic volume change and TV. Moreover, these factors have high positive correlation with body weight. The clinical paradox mentioned above in Introduction Section could be well explained by these observations. The OSAHS patients with large MPCA might also possess higher body weight and larger value of 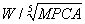 . Thus, these patients suffered from more severe clinical symptoms and showed higher AHI than those with small MPCA, lower body weight, and smaller value of
. Thus, these patients suffered from more severe clinical symptoms and showed higher AHI than those with small MPCA, lower body weight, and smaller value of 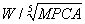 (Figure 4).
(Figure 4).
AT/ST is an index that reflects the total time of upper airway collapse and apnea during the entire sleep period. The data in this study showed significant correlation between AT/ST and the new comprehensive index. The correlation was also stronger than those with body weight or MPCA. T90 is an effective index because it reflected the hypoxia level caused by upper airway collapse during the entire sleep period. Furthermore, the correlation between T90 and the new comprehensive index was significant. The correlation coefficient was larger than that with body weight or MPCA. These data suggested that both AT/ST and T90 were also affected by the joint effect of weight and MPCA. Therefore, the new comprehensive index could also be used as the objective indicator of these parameters.
Unexpectedly, no significant difference was found in the LaSO2 values between Group A and Group B. LaSO2 showed weak correlations with body weight, MPCA, and the new comprehensive index, indicating other important influence factors might be involved in LaSO2. These factors might include lung function, oxygen carrying capacity, and oxygen consumption rate etc.
Although the new parameter had positive correlations with AHI, thoracic volume change or TV might play roles in apnea during sleep. However, the TV data were neither recorded nor analyzed. Additionally, the method of obtaining TV from body weight is more suitable for patients with normal BMI. The correlations between TV and weight of OSAHS patients with larger BMI are weaker than those of persons with normal BMI. Thus, the next study plan involves a survey on the correlation between apnea and the accurately measured TV.
The pathogenesis of the upper airway collapse under negative pressure is complicated. No ideal mathematical models or formulas for upper airway flow-negative pressure relationship are available. A simple combination (weight as numerator and MPCA as denominator) did not improve the correlation with AHI effectively. Thus, we used the nth root of MPCA as a different denominator. The correlation coefficients between the new comprehensive indices and all of the sleep-associated monitoring indices improved significantly. However, the increasing trend was limited. The values of the correlation coefficients gradually increased and subsequently decreased. The 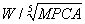 was only a turning point.
was only a turning point.
We also found that the value of the coefficient of determination of Equation B was significantly larger than that of the coefficient of Equation A, suggesting that body weight and MPCA jointly influenced the upper airway collapse in a more complicated manner. Thus, these parameters should not be used as two individual factors in analyzing the correlation with OSAHS.
Although the new comprehensive index had an ideal significant correlation with AHI, the effectiveness of 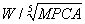 cannot be determined because of the lack of support in terms of confirmed mathematical models. Nevertheless, this new comprehensive index deserves attention because it is capable of elucidating the pathogenesis of OSAHS effectively. In this relation, more related factors should be combined to define pathogenesis more efficiently and obtain more effects and accurate predictors.
cannot be determined because of the lack of support in terms of confirmed mathematical models. Nevertheless, this new comprehensive index deserves attention because it is capable of elucidating the pathogenesis of OSAHS effectively. In this relation, more related factors should be combined to define pathogenesis more efficiently and obtain more effects and accurate predictors.
Compared with the single factor, the two-factor comprehensive index has the capability to reveal the mechanism of obstructive sleep apnea more effectively. Combination of body weight and MPCA is a new and simple method to assess the severity of OSAHS to some extent. The data of MPCA and body weight can be obtained easily as well.
We would like to thank Xiaorong Zhou and Chan Meng for their technical assistance.
There are no conflicts of interest.