Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research Article - (2020)Volume 10, Issue 4
Background: Premenstrual Syndrome (PMS) is a very common disease in females, and safe treatments are stillneeded.
Objective: To treat symptoms of PMS using a combination of natural products at low dosages following the conceptsof Physiological Modulation.
Methods: Twenty two female affect by PMS were admitted and treated with a combination consisting of calcium,Vitamin D, lycopene, astaxanthin, and citrus bioflavonoids (Formula A28) in comparing with placebo. Twotablets/day were given at the moment of symptoms appearance and for the following three days.The scoring of somatic and behavioral symptoms (in total 32) was applied using a scale between 0 (no symptom) to 4(maximum expression of the symptom). The main variable to be measured was the sum of the scores (total score).
Results: Formula A28 was effective in reducing by 75% the total score (t-test p<0.05) and each of the symptoms(somatic and behavioral) was significantly reduced (p<0.05 U Mann Whitney test) from the 63% (anxiety) up to 96%(indecision). Colic pain was also consistently reduced (83%).
Conclusion: The approach to reduce symptoms of PM Susing Physiological Modulators was found to be effective.
Premenstrual syndrome; Physiological modulation; Somatic symptoms; Behavioural symptoms
Premenstrual Syndrome (PMS) consist of psychological, behavioral, and physical symptoms that occur during the late luteal phase of the menstrual cycle and disappear at the onset of menstruation [1].
As much as 25% of menstruating women report moderate-to severe premenstrual symptoms. And in approximately 5% of the cases are reported as severe [2].
The suggested etiology of both includes abnormal neurotransmitter responses to normal ovarian functions, hormonal imbalance, sodium retention, or nutritional deficiencies [3].
There is evidence of a relationship between the syndrome and changes in certain progesterone metabolites (pregnenolone and allopregnanolone) during the menstrual cycle which may interfere with neurotrasmitters, especially Gamma-Aminobutyric Acid (GABA) and serotonin which are important regulator of stress, anxiety, and alertness. These aforementioned metabolites act as positive modulators of the GABAergic system in the brain and also in the gastrointestinal tract and make less receptive the GABAA receptor.
Depletion of serotonin levels is associated with anxiety and depressive symptoms, since the Selective Serotonin Uptake Inhibitors (SSRI) are found to be active in PMS, and has been suggested that serotonin increase the sensitivity to progesterone.
Pharmacological treatments of PMS has included antidepressants (selective serotonin inhibitors, SSRIs) and other psychotropic agents, diuretics, progesterone, GnRh agonists, hormonal therapy such as estrogen therapy, combined oral contraceptive, pyridoxine, ethinylestradiol and dosperidone, and synthetic androgen and gonadotropin inhibitors [4]. However, more women were found to use non-pharmaceutical approaches including dietary changes, exercise, cognitive behavioral therapy, and complementary alternative medicine that sometimes were shown to be effective [5]. There is an aspect that has not been analyzed carefully and belongs to the oxidative stress condition during the PMS. Despite some author was underlining this condition as an imbalance between oxidant/antioxidant status [6] conflicting results were found by others but none was determining the activity of compounds or antioxidant formulations. A particular combination of antioxidants was shown to reduce the oxidative stress during menstrual cycle in healthy women without PMS [7]. The aim of our research was to determine the modification of PMS symptoms administering a combination of products (F) with natural anti-inflammatory and antioxidant activity, calcium and vitamin D at low dosages as typical for Physiological Modulation approach [8].
Admission criteria
Women between 20 and 35 years were admitted presenting somatic and behavioral symptoms as reported in Table 1.
| Somatical symptoms | Behavioral symptoms |
|---|---|
| Colic pain | Irritability |
| Bloatedness | Emotional tension |
| Brest tenderness | Anxiety |
| Intestinalmodification | Mood swing |
| Body weightincrease | Depression |
| Hot flashes | Aggression |
| Acne | Anger |
| Libido reduction | Fatigue |
| Back pain | Confusion/loss of concentration |
| Foodcraving | Lethargy |
| Dizziness | Sadness |
| Headake | Indecision |
| Thirst | Paranoia |
| Nausea | Over Sensitivity |
| Clumsiness | Loss of motivation |
| Fluidretention | Cryingeasily |
Table 1: Somatic and behavioral symptoms of premenstrual syndrome.
Exclusion criteria
Subjects suffering from any cancer, chronic diseases such as Alzheimer ’ s disease, depression, anorexia, paranoia, Chron ’ s disease, bowel irritable syndrome, allergy or intolerance were not admitted to the trial.
Other diseases such as hypertension, dyslipidemia were not within the exclusion criteria provided that the therapy in place was, effective, safe, and established by at least 3 months.
Women under oral contraceptive treatment were not excluded only in case that the treatment was safe and identical for at least 6 months.
Patients
Twenty-two women between 14 and 45 years (all but 4 were health professionals nurse or doctors), were admitted presenting somatic and behavioral symptoms of PMS as reported in Table 2 [2].
| Variable | Mean | SD |
|---|---|---|
| Age (years) | 34 | 11.6 |
| Age of menarche (years) | 13 | 1.3 |
| Duration of menstruation (days) | 5 | 1.4 |
| Frequency | ||
| School University degree/total | 6/22 | |
| School Bachelor/total | 12/22 | |
| Activity: professional/total | 18/12 | |
| Activity: house wife/total | 0/22 | |
| Students | 4/22 | |
| Smoking/total | 0/22 | |
| Concomitant therapy: oral contraceptive | 16/22 | |
| Concomitant therapy; other | 2/22 | |
| Previous therapy for premenstrual syndrome | 18/22 |
Table 2: General characteristics of the patients: mean values and SD, and frequency.
Subjects were instructed to fill up a daily questionnaire relative to the symptoms, by mean of a semi-quantitative scale scoring from 0 to 4 (see later in experimental procedure) to be completed during 3 subsequent menstrual cycles.
At least 5 somatic and 5 behavioral symptoms were necessary to be admitted to the trial.
Institution
The trial was conducted under the supervision of Irwin Labs (Spoltore-PE Italy).
Experimental procedure
The data were recorded during three subsequent menstrual cycles, and only those women reporting similar symptoms in the first two evaluations were admitted. The criteria used for admission were such that only 1 point of difference between the first two score evaluations (baseline and placebo) for any of the symptoms was accepted. In case of a scoring difference of >1 the subject was not admitted to the trial.
Variables measurement
Three sets of measurements for the variables were requested: The first consisted of the baseline evaluation, in the day of the symptom appearance, and was done by the investigators together with the participants; the second evaluation was done directly by the patients only after the treatment with placebo.
The third evaluation was done by the patient only during the treatment with the active formula (F).
The highest scoring only was used for the evaluation, no matter about the day (1 to 3) of appearance.
Treatments
The month immediately after the baseline evaluation consisted of placebo in the quantity of 2 cps, to be taken together immediately before sleeping and at least two hours after the evening meal. The treatment was continued for 3 days before the expected menses.
The second treatment was immediately following next month consisting of 3 days, where the active formula (A28) was given in the amount of 2 cps/day, to be taken together before sleeping. The patients were instructed to take F at least two hours after the evening meal. Two boxes containing 10 cps (placebo or A28) were given to each participant. The capsule of both treatments, placebo and F, were identical for color and weight.
Main variables
The main variables were the sum of the score of both somatic and behavioral symptoms, while all the other variables were considered as ancillary variables.
This study was based on a heuristic hypothesis, since no data were available to calculate the test power.
The average values and dispersion measures were calculated for each variable following the placebo and F treatments. The differences between treatments (placebo and A28) were tested using the Test U (Mann-Whitney). Limited to the sum of the somatic and behavioral symptoms the t test was calculated.
The JMP15 Pro of SAS Institute (2020) was used for the calculations.
Compliance
The compliance was consisting of the counting of residual cps both for placebo and active formula.
All the 22 cases concluded the experience, and no side effect was declared. The patients ’ general characteristics are summarized in Table 2.
The level of education was quite high, since most of the patients were medical professional, and 4 cases only were young students.
Data relative to the scoring are summarized in Table 3. Almost for all the symptoms the highest scores were appearing in the first day before menses.
| Period | Placebo | After A 28 | %Inhibition | ||
|---|---|---|---|---|---|
| Measures | Mean | SD | Mean | SD | |
| Somatic variables | |||||
| Colic type pain | 2.9 | 0.75 | 0.5Ɏ | 0.50 | 83 |
| Bloatedness | 1.7 | 0.77 | 0.4 Ɏ | 0.50 | 76 |
| Breast tenderness | 2.4 | 0.79 | 0.8 Ɏ | 0.49 | 66 |
| Intestinal modifications a | 1.9 | 0.87 | 0.7 Ɏ | 0.47 | 65 |
| Body weight increase | 0.5 | 0.67 | 0.1 Ɏ | 0.27 | 86 |
| Hot flashes | 2.2 | 0.80 | 0.8 Ɏ | 0.49 | 63 |
| Acne b | 1.3 | 1.04 | 0.5 Ɏ | 0.71 | 62 |
| Libido reduction | 2.0 | 1.50 | 0.7 Ɏ | 0.60 | 64 |
| Back pain | 2.2 | 0.50 | 0.5 Ɏ | 0.51 | 77 |
| Food craving | 1.0 | 0.82 | 0.5 Ɏ | 0.57 | 52 |
| Dizziness | 1.6 | 0.73 | 0.1 Ɏ | 0.33 | 93 |
| Headache | 2.6 | 0.50 | 0.5 Ɏ | 0.58 | 82 |
| Thirst | 1.4 | 0.67 | 0.5 Ɏ | 0.51 | 65 |
| Nausea | 1.5 | 0.74 | 0 Ɏ | 0.20 | 97 |
| Clumsiness | 1.0 | 0.76 | 0.1 Ɏ | 0.27 | 92 |
| Fluid retention | 1.0 | 0.69 | 0.3 Ɏ | 0.53 | 73 |
| Total score somatic | 27.2 | 5.27 | 6.9 c | 2.71 | 75 |
| Behavioral variables | |||||
| Irritability | 2.5 | 0.74 | 0.8 Ɏ | 0.43 | 69 |
| Emotional tension | 2.3 | 0.63 | 0.7 Ɏ | 0.56 | 71 |
| Anxiety | 2.1 | 0.64 | 0.8 Ɏ | 0.40 | 63 |
| Mood swing | 2.0 | 0.79 | 0.8 Ɏ | 0.40 | 70 |
| Depression | 2.0 | 0.98 | 0.2 Ɏ | 0.46 | 92 |
| Aggressiveness | 1.4 | 1.18 | 0.4 Ɏ | 0.57 | 73 |
| Anger | 2.2 | 0.75 | 1.1 Ɏ | 0.48 | 52 |
| Fatigue | 2.4 | 0.73 | 0.7 Ɏ | 0.55 | 71 |
| Loss of concentration | 1.9 | 0.94 | 0.4 Ɏ | 0.50 | 77 |
| Lethargy | 1.4 | 0.91 | 0.2 Ɏ | 0.37 | 89 |
| Sadness | 2.3 | 0.89 | 1.0 Ɏ | 0.20 | 55 |
| Indecision | 1.9 | 0.75 | 0.1 Ɏ | 0.27 | 96 |
| Paranoia | 1.8 | 1.11 | 0.1 Ɏ | 0.43 | 93 |
| Over sensitivity | 2.0 | 0.76 | 0.8 Ɏ | 0.37 | 58 |
| Loss of motivation | 2.2 | 0.91 | 0.2 Ɏ | 0.40 | 91 |
| Crying easily | 1.7 | 0.99 | 0.1 Ɏ | 0.33 | 93 |
| Total score behavioral | 32.7 | 10.91 | 8.3 c | 75 | |
Table 3: Scores averages (mean and SD) and percentages of inhibition following A 28 treatment.
The main observations that can be drawn from the study are the following
The total scoring following placebo and after the treatment with F were significantly different (t test p<0.001) in favor of F accounting for an average reduction of 75%.
Before the treatments with A28, the total scoring was higher for behavioral compared to somatic symptoms (respectively 32.7 ± 10.91 and 27.2 ± 5.27) and the differences were statistically significant (t test p<0.05).
All the variables were affected following F treatment with different efficiency in reducing symptoms from 55% (sadness reduction) up 97% (nausea).
The most affected symptoms were dizziness, nausea, clumsiness, depression, indecision, paranoia, loss of motivation, and crying easily, for which the reduction was > 90% [9,10].
Several reviews exist of pathophysiological hypothesis of PMS
Most of the studies do not identify consistent abnormalities in hormones of the hypothalamic-pituitary-gonadal (HPG) axis, although a few studies have suggested altered luteinizing hormone (LH) pulse [11,12].
As well studies have, not identified abnormalities in thyroid hormones, cortisol, prolactin, glucose, ß-endorphins, vitamins or electrolytes [13,14]. In general is thought that premenstrual symptoms occur as a result of a differential sensitivity in the mood-perturbing effects of gonadal steroid fluctuation in women with PMS and PMDD. It is probable that the etiology is multifactorial and in part genetically determined and due to neurotransmitter and neuropeptide differential sensitivity [15].
Although the specific neurotansmitter, neuroendocrine and neurosteroid abnormalities determining the syndrome are not known, serotonin, norepinephrine, γ-aminobutiric acid (GABA), allopregnandolone (ALLO) which is a metabolite of progesterone that acts at the GABAA receptor), endorfins and factor involved in calcium homeostasis may be all involved.
It may be interesting consider that receptors of any type (e.g for serotonin, norepinephine, GABA) are located primarily on the cellular membranes. The membrane functions in general belong to the oxidative condition of the membrane itself, and the receptor is maintained in place with the normal function depending upon the membrane structure around and within the receptor. The membrane around and within the receptor (rafts) can have a differential sensitivity to the oxidative stress (OS) depending upon the quantity of double bonds in the lipids constituting the phospholipids (PL) and the antioxidant reserve available in the cellular system. Lipids rafts consisting of cholesterol/sfingo lipids micro domains are involved in protein traffic, formation of signaling complexes and are abundantly represented of several postsynaptic dendrites [16].
This means that a neuroamine receptor (e.g for serotonin, dopamine) may be more sensible to the oxidation than an estrogenic receptor or vice versa.
The consequence of this is that even a mild oxidation in absence of an adequate antioxidant capacity can trigger the dysfunction of certain receptor only, whereas a massive oxidation generates a complete deregulation of all the receptors.
In any case the oxidative stress tend to be an explosive process, and also a mild oxidation that starts in one point/area (lets us call it “guilty amplifier”) may be spread out rapidly involving all the proximal areas. This means that is possible to stop the explosive process just protecting from oxidation this area and the relative symptoms will not emerge. In case of massive oxidation the possibility to overcome the problem is very hard. The simple scheme in Figure 1 may explain the mechanism.
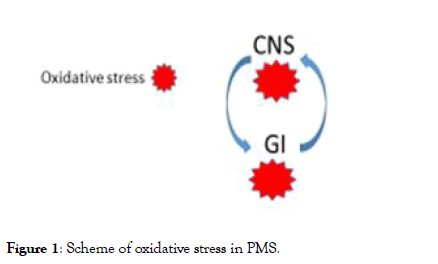
Figure 1: Scheme of oxidative stress in PMS.
Description of Figure 1: The oxidative stress can be generated in the CNS or in the GI. In both cases it is transmitted from one department to the other: its control even in one department only can avoid the diffusion to the other.
One example of this ambivalent condition is the use of oral contraceptives in the control of PMS. The combined hormonal contraception (CHC) was shown to have beneficial effect [17] on the syndrome.
Oral contraceptives increases substantially the OS condition, however in most of the cases they do not cause any evident damage in normal women.
In fact, OS following CHC is not causing any particular symptom, a part of and the risk of venous thrombosis and some vague complains, meaning that the hormonal increase determined by the estrogens and/or progestins daily administration do not cause those symptoms which are characteristic of PMS/PMDD, despite the massive increase of OS.
One of the reason could be this increase is counterbalanced by the concomitant overproduction of the antioxidant reserve. In other terms all the system seems to be over expressed and balanced.
Despite CHC sometimes is effective in the treatment of PMS, in many cases it fail in the control of the symptoms. As known the progestogens contained in some hormonal contraceptives are metabolized (into pregnenolone and allopregnanolone) which modify and activate the GABAergic system increasing the central GABA-mediated inhibition.
The lack of the activity of CHC on PMS indicates that the syndromes are not related to the dysregulation of individual neurotransmitters and belongs to a more intricate mechanism.
The GABAA receptor
The GABAA receptors are the major inhibitory neurotransmitter receptors in the mammalian brain, widely present particularly in the striatum, mesolimbic, cortex, hypothalamus, midbrain.
Particularly important PMS are those located in the amygdala. GABAA receptors open channels for Cl that lead to an influx of ions that hyperpolarizes the neuronal membrane and makes less likely to generate action potentials to stimulate output neurons to firing from the amygdala to the frontal cortex.
In the amygdala when the excitatory signals (EPSPs or excitatory post synaptic potential) caused by fear or stress will be inhibited, a marked decrease in anxiety and a greater feeling of calm take place.
The GABAA receptors consist of 5 subunits 2α1, 2ß2, 1γ2 coupled around a central ion pore [2,3] and located transversally on the membrane (Figure 2).
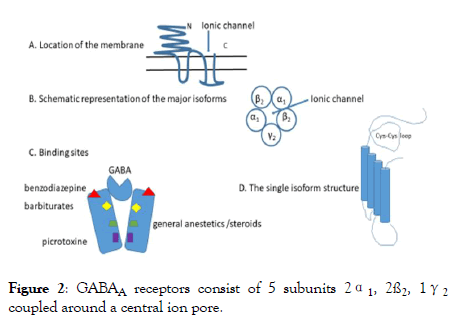
Figure 2: GABAA receptors consist of 5 subunits 2α1, 2ß2, 1γ2 coupled around a central ion pore.
Description of Figure 2: A. The receptor has a large dominion outside of the membrane with the aim of stabilize the structure and maintain the ionic channel in place.
B. The channel proteins belong to genes located into four gene clusters (chromosome 4, 5, 15 and X) which determine the various isoforms, α, β, γ each with different dispositions among them, and inside the same protein. The most represented seems the pentameric complex arranged as γ2 β2 α1 β2 α1.
C. Binding sites are many, located in different part of the receptors and each site determine the opening of the channel allowing the Cl- inflow and the modification of membrane potential.
D. The single isoform is characterized by proteins containing in the external part a loop that is maintained by two cysteins, stabilized by a double bond which can be easily oxidized [18]. An oxidation of this bond makes the monomeric protein inefficient and do not allow the appropriate function of the ionic channel.
This aspects make clear that OS may compromise directly (receptor per se) or indirectly (rafts) the neuronal firing and inhibitory neurons can be more sensible than stimulating neurons [19-21].
Bioflavonoids have been found to interact with ionotropic GABA receptors, acting via multiple binding size and can be one of the possibilities to modulate their activity [22].
Calcium sensing receptors
Extracellular fluid (ECF) Ca concentration is maintained within a narrow range in normal individuals since any change in its entry into the EFF is rapidly matched by an identical change of urinary excretion. An adequate secretion of parathyroid hormone (PTH is required for this adaptation and Ca sensing receptors (CaSR) are fundamental to make efficient this minuteto minute control. However, Many CaSRs (Figure 3) are present thorough the body, in kidney, bones, skin, stomach and colon [23].
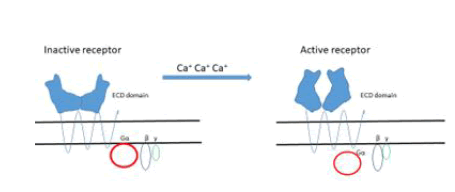
Figure 3: The binding of Ca+ ions to the receptor is of the type“Venous flytrup”.
Description of Figure 3: Activation results in conformational changes resulting in signal transduction through the complex Gα part of the inhibitory/activatory glycoprotein cluster G α β γ.
The ECD domain in humans contains 612 amino acids with the presence of 17 cysteins that form four disulfide bonds stabilizing the quaternary structure which is fundamental for the protein functionality.
Cysteins are very sensible to oxidation, and an appropriate antioxidant protection is needed to maintain the efficiency of the CaSR receptor.
For what concerns the gastrointestinal tract in humans, CaSR in humans are present in the stomach, in the small and large intestine and in the colon.
In the stomach CaSR receptor are localized to the basolateral membrane of surface epithelial cells, in the antrum and to a lesser extent in the acid-secreting glands in the fundus; in both cases they control the acid secretion.
In the small and large intestine they are localized on the basal membrane of the intestinal crypt and villiepithelial cells.
In the colon surface cells are responsible for absorption whereas in the crypt are responsible for secretion and motility through the smooth muscles nerve plexus.
According to the concept of Physiological Modulation the disease was treated using low dosages of products aimed to reset the GABA receptors, CaSRs, inflammation, and oxidative stress as reported in Table 4.
| Ingredient | Dose | Unit | Activity |
|---|---|---|---|
| Vitamin D3 (cholecalciferol) | 1.25 | μg | Calcium absorptions |
| Calcium carbonate | 209 | mg | Calcium receptors (CARS) modulation in the GI |
| Calcium lactate | 143 | mg | Calcium availability |
| Lycopene | 1.0 | mg | Reduction of lymphocytes reactivity as inflammatory triggers |
| Astaxanthin | 0.25 | mg | Antioxidant activity for central GABA receptors |
| Citrus flavonoids | 33.3 | mg | Antioxidant, anti-inflammatory activity |
Table 4: CaSRs, inflammation, and oxidative stress.
The use of 2 cps allows doubling the quantities, which still are substantially low. The combination of products was found very active, confirming that the approach following MF concepts was clinically valid.
The treatment of PMS with a formula based on the concepts of Physiological Modulation was shown to be very effective in reducing somatic and behavioral symptoms.
UC was conceiving the trial, JRHS and GB were treating and evaluating the patients, UC was evaluating the data and wrote the text.
No funding was received for the trial, which was conducted as investigators initiative.
Brava Company (Mexico City) was providing the placebo, and the product.
The study was approved by the Ethics Committee IAPS (International Agency for Pharma Supplements) No 12 of May 2019, and written informed consent was obtained by all the participants.
No conflict of interest. A28 is protected with a patent filed by a Mexican Company
Citation: Umobert C, Santos HJR, Belcaro G (2020) Premenstrual Syndrome (PMS) treatment with Physiological Modulators. Gynecol Obstet (Sunnyvale) 10:523. doi:10.35248/2161-10932.2020.10.523
Received: 27-Feb-2020 Accepted: 06-Mar-2020 Published: 15-Mar-2020 , DOI: 10.35248/2161-10932.20.10.523
Copyright: © 2020 Umobert C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.