Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2019)Volume 8, Issue 1
The research includes the preparation of new azo-barbiturate dyes derived from schiff bases and repellency bases by means of double-reaction of repellant bases with different phenolic compounds. These compounds were diagnosed using infrared techniques, ultraviolet-visible radiation, NMR proton and thin layer chromatography. All barbiturate dyes had absorption between (273-310) nm and showed hypsochromic shift from the corresponding azo dyes. The crystal structures of barbiturate dyes indicated that barbiturate ring is sterically hindered by phenol rings.
Barbituric acid; Schiff base; Azo dyes; Azumethin derivative
Barbiturates derived from an acidic basis are barbituric acid and are characterized as medium to low acidity compounds with acidic function [1,2]. These compounds are not insoluble in water and have been prepared to overcome this characteristic by reacting with sodium ions, which is prepared in the form of salts and becomes soluble in water [3,4]. Several barbiturates were derived by substituting the roots in their basic construction, barbituric acid, which scientists agreed to number their atoms [5]. These derivations include the radical substitution of ethers where hydrogen atoms on carbon 5 resulted in the discovery of the barbiton compound [6]. The replacement of a vinyl root by the location of one of the ethel radicals on carbon 5 (in the barbiton compound) led to the discovery of phenobarbeton, which is characterized as antimicrobial and muscle dysfunction [7,8]. Replace the sulphur element with the oxygen element on carbon 2 and replace the side chain with the place of one of the ethyl radicals on carbon 5 (in the barbiton compound), resulting in the thiopantone compound [9]. The radical replacement of the instance of the two hydrogen atoms associated with the azotone 1, 3 and the replacement of cyclohexanyl and the place of one of the ethyl radicals on carbon number 5 resulted in the acquisition of sodium hexoparbeton [10]. The latter two have the fastest impact, i e, rapid anesthesia and short impact duration [11]. Schiff bases a group called azomethine (C=N) [12-14]. Also, schiff bases derived from aldehyde condensation with primary amines are called al-deamins. In the so-called schiff bases derived from the condensation of ketones with the primary amines are called al-katamines [15]. The stability of schiff bases depends on the type of amine and the type of aldehyde or ketone used. The bases of the schiff prepared from the aromatic ketone and the aromatic amine are the most stable between the schiff bases. This is due to increased stability by resonance [16]. Azumethin dyes are of great importance and wide use in industries Paper, coloured reagents for food and cosmetics [17-21]. Azumethin compounds are also bioactive, used as antimicrobial agents, cancer and others [22-25].
All compounds were purified by re-crystallization followed by thin layer chromatography and modern spectral techniques. As for melting points, it was measured using Staurat MP/MP3. Infrared spectra were recorded using a spectrometer (Shimadzu FT-IR 8400 S) with a potassium bromide tablet. Absorption spectra were measured in ethanol using spectroscopy (Shimadzu UV-Vis. 1600) at Qadisiyah University - Faculty of Education. The proton NMR spectrum was taken using a BRUKER (300 MHZ) spectrometer in Jordan-Al-Bayt University. Chromatography was measured using Silica Gel TLC plates 60 F250.
Methods of preparation
The general method for-preparing the base of azomethin: In R.B.flask (100 ml), put 0.02 ml of pyrimidine derivative and add 25 mL of pure ethanol and then (1 ml) gla. acetic acid, and gradually add (0.06 mol) aromatic amine with (20 ml) pure ethanol and then the mixture reflux for 2 hr. The reaction was followed by chromatography of the thin layer. After heating, the reaction mixture was cooled, the precipitate was then filtered and dried and then recrystallized using ethanol [26-28].
The general way to prepare azo pigments
Preparation of diazione salt sol: In a glass baker, prepare a solution consisting of 10 mL cooled water and 10 mL concentrated hydrochloric acid and with continuous stirring (0.02 mL) of the prepared schiff base in the first part (A) with cooling in the ice bath (0-5) M, then add to it a coolant solution consisting of (0.06 mol) sodium nitrite+(5 ml) water. In a second glass baker and within the ice bath, a solution consisting of dissolving (0.06 mol) phenolic compound with (15 ml) sodium hydroxide solution (10%). After continuous stirring in the ice bath, add the diazonium salt solution and drops to the coupling solution. After the addition of the mixture, stir the reaction mixture for 15 minutes in the ice bath. The deposited precipitate is washed with water and then recrystallized using ethanol.
This research included two steps
The first step was to prepare a new schiff base (A) by reacting the condensation between barbituric acid and the 1,4-phenylene diamine compound (Scheme 1).
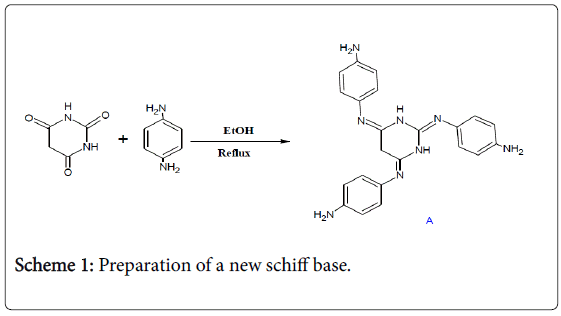
Scheme 1: Preparation of a new schiff base.
The second step involved the preparation of new azo dyes through the interaction of schiff base prepared in the first step using diazotization reaction with several phenolic compounds (Scheme 2). Eight new azotecin derivatives were obtained in compounds. Table 1 represents all physical properties of prepared derivatives. Table 2 represents all the spectral properties of the prepared compounds. Spectra FT-IR shows in Figure 1 [29-31].
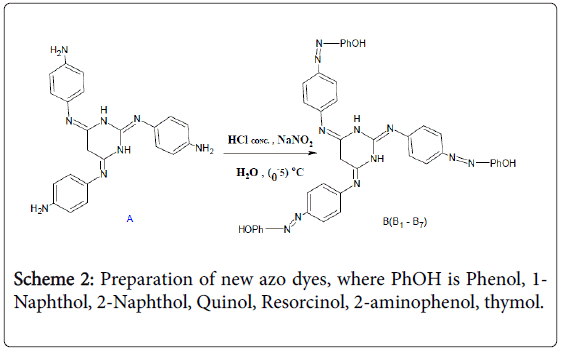
Scheme 2: Preparation of new azo dyes, where PhOH is Phenol, 1- Naphthol, 2-Naphthol, Quinol, Resorcinol, 2-aminophenol, thymol.
| Comp. | Phenol | M.Str. | M.wt.(g.mol-1) | Yield | M.P. (°C) | Color |
|---|---|---|---|---|---|---|
| A | ------- | C22H22N8 | 398.39 | (0.3,80) | 190-192 | D. Orange |
| B1 | Phenol | C40H31N11O3 | 713.74 | (0.4,60) | 205-206 | Yellow |
| B2 | 1-Naphthol | C52H37N11O3 | 863.92 | (0.5,70) | 257-258 | L. yellow |
| B3 | 2-Naphthol | C52H37N11O3 | 863.92 | (0.6,68) | 271-273 | Orange |
| B4 | Quinol | C40H31N11O6 | 761.74 | (0.4,58) | 232-234 | Brown |
| B5 | Resorcinol | C40H31N11O6 | 761.74 | (0.4,65) | 230-232 | D. yellow |
| B6 | 2-Amino phenol | C40H34N14O3 | 758.78 | (0.3,55) | 240-241 | L. orange |
| B7 | Thymol | C52H55N11O3 | 882.06 | (0.2,50) | 265-267 | D. brown |
Table 1: Physical properties of prepared derivatives (L: Light, D: Dark).
| Comp. | Phenol | λmax | FT-IR (cm−1) KBr disk | ||||
|---|---|---|---|---|---|---|---|
| EtOH | |||||||
| - NH | -C=N | N=N | C–O | -OH ring | |||
| A | ------- | ------- | 3373 | 1635 | ------- | 1284 | ------- |
| 1 | Phenol | 273 | 3345 | 1631 | 1523 | 1290 | 1474 |
| 2 | 1-Naphthol | 288 | 3362 | 1614 | 1522 | 1294 | 1478 |
| 3 | 2-Naphthol | 290 | 3359 | 1618 | 1518 | 1293 | 1476 |
| 4 | Quinol | 275 | 3387 | 1616 | 1522 | 1291 | 1475 |
| 5 | Resorcinol | 278 | 3395 | 1617 | 1524 | 1294 | 1476 |
| 6 | 2-Amino phenol | 295 | 3372 | 1620 | 1526 | 1296 | 1480 |
| 7 | Thymol | 310 | 3422 | 1623 | 1530 | 1298 | 1481 |
Table 2: Spectral properties of prepared derivatives.
Figure 1: Spectra FT-IR of compounds.
Spectrometry (1H-NMR) of prepared derivatives 1H NMR (300 MHz, CDCl3, δ/ppm)
Comp. A: 2.96 (d, 2 H, J=14.8 Hz), 3.59 (d, 2 H, J=14.8 Hz), 6.98 (m, 7H), 7.06 (s, 5 H), 7.17 (m, 7 H).
Comp. B: 3.31 (s, 2 H), 6.96-7.03 (m, 8 H), 7.32 (m, 8 H), 7.50 (q, 7 H, J=6.4 Hz), 7.60 (dd, 7 H, J=7.0, 1.7 Hz).
Comp. C: 3.29 (s, 2 H), 6.96 (dd, 3 H, J=8.6, 1.9 Hz), 7.22 (m, 7 H), 7.50-7.77 (m, 18 H), 7.81-7.88 (m, 3 H), 8.18-8.27 (m, 3 H).
Comp. D: 3.29 (s, 2 H), 6.98 (d, 2 H, J=8.8 Hz), 7.04 (d, 1 H, J=8.6 Hz), 7.22 (m, 7 H), 7.50-7.70 (m, 15 H), 7.76 (dd, 1 H, J=8.2, 1.6 Hz), 7.86-8.00 (m, 8 H), 8.11 (dt, 1 H, J=7.8, 2.1 Hz).
Comp. E: 3.23 (s, 2 H), 6.45-6.48 (m, 4 H), 6.83-6.87 (m, 4 H), 7.23 (m, 3 H), 7.31 (s, 11 H), 7.44-7.50 (m, 4 H), 7.67 (d, 3 H, J=8.1 Hz).
Comp. F: 3.08 (d, 1 H, J=15.3 Hz), 3.38 (d, 1 H, J=15.3 Hz), 6.45-6.48 (m, 4 H), 6.83-6.87 (m, 4 H), 7.23 (m, 3 H), 7.31 (s, 11 H), 7.44-7.50 (m, 4 H), 7.67 (d, 3 H, J=8.1 Hz).
Comp. G: δ 3.21 (s, 3 H), 6.48 (d, 1 H, J=1.8 Hz), 6.55 (d, 3 H, J=1.1 Hz), 6.68-6.71 (m, 4 H), 7.15 (m, 6 H), 7.21-7.31 (m, 12 H), 7.54 (d, 4 H, J=8.2 Hz).
Comp. H: 1.32 (d, 20 H, J=6.8 Hz), 2.08 (s, 3 H), 2.13 (s, 6 H), 3.27 (s, 2 H), 3.38 (h, 3 H, J=7.1 Hz), 6.68 (s, 2 H), 6.80 (s, 1 H), 7.20-7.29 (m, 7 H), 7.46-7.51 (m, 9 H), 7.63 (s, 1 H).
The NMR spectrum of the azoketimines indicates that there are several overlapping electronic environments of the prepared compounds due to the diversity of the active groups found in these compounds in that they are driving or drawing aggregates (such as azomethane, hydroxyl, amine and alkyl groups), in addition to the abundance and diversity of hydrogen atoms (6.60 ppm) refers to the adjacent protons of the base aromatic ring, whereas the signal (6.80 ppm) belongs to the opposite protons of the aromatic compound. The opposite protons of the aromatic amine compound, close to the azomethin group, show a clear signal with double bursts of (7.81 ppm). For the latter, they are due to the opposite protons of the base aromatic ring, especially near the fivering. For the phenol compound, one of the most significant signs of the characteristic resonance characteristic is the signal (6.99 ppm) of the corresponding phenol ring protons, which at the same time are protons adjacent to the other phenol loop protons, causing double and double fissures. As for the naphthol dye, the most important signals are measured between the extended values (ppm 7.09-8.03), which are related to the protons of the alpha-naphthol ring, which are close to the hydroxyl driving group and the torso-tzo group, Hydrogen atoms. As for azumethane and pyrethylene, the presence of the high-density electron groups creates electronic environments that obscure or reduce the appearance of signals and thus reduce their measured values or observations. This is observed for these compounds, the most important of which are the measured values (7.17-6.91 ppm), which is due to the proton ring between the hydroxyl driving groups. The characteristic and phenolic value of the phenolic compound is a signal between (7.18-6.80 ppm), which comes from protons confined between the iso group and the amino and hydroxyl groups. The most recent of these thymol has several distinctive signs, the most important of which is the signal (3.23 ppm) of proton, the isopropyl group, which is affected by the high electronic environment of the two kinetic groups, as well as the signal (1.96 ppm) of the three methyl groups of the thymol (1.18 ppm). The signal (6.99 ppm) belongs to the thymol group, which is bound to the methyl and hydroxyl groups.
The main objective of this research is to prepare new azotecins containing several phenolic derivatives using one of the important organic reactions, which is the reaction of azuna. The classical and modern techniques were used in the diagnosis of prepared compounds such as melting grades, chromatography, Ultraviolet- visual radiation, proton spectroscopy and NMR spectroscopy. The percentages of derived derivatives ranged approximately and mostly between the mean to the good. We hope that our research will be done in the form of new azotecin dyes used in some industrial and applied fields such as dyes, various medicines, cosmetics, colored reagents, analytical chemistry guides [32-36].
Citation: Kazim AC and Kadhim AJ (2019) Preparation and Diagnosis of New Azo-Barbiturate Dyes. Organic Chem Curr Res 8:196.
Received: 08-Jan-2019 Accepted: 23-Jan-2019 Published: 30-Jan-2019
Copyright: © 2018 Kazim AC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.