Pharmaceutical Analytical Chemistry: Open Access
Open Access
ISSN: 2471-2698
ISSN: 2471-2698
Research Article - (2017) Volume 3, Issue 2
The present report is concerned with the synthesis novel Schiff base ligand by condense 6-Amino Penicillinic acid with Salicyldehyde. The ligand was characterized by 1H-NMR, 13C-NMR, mass spectrometry, UV-Vis and FT-IR studies. The ligand have got more than one moiety which are capable of chelation, therefore complexes of Co(II), Ni(II), Cu(II), and Zn(II) were prepared. These complexes were characterized by UV-Vis, FT-IR, magnetic susceptibility and Molar conductivity studies. All these techniques indicate 1:2 stoichiometry of synthesized complexes. In all the complexes, Schiff base ligand acts as a tridentate ligand. Finally, the ligand and complexes have been screened for their antibacterial activity against four types of bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus facials and Proteus mirabilis) then it isolated from different classes of ulcerative infections while L showed to be tridentate ligand with single negative charge through three active groups, L has been associated with metal ions to form the claw complexes to get octahedral shape. The prepared ligand and its complexes illustrated a good inhibitory ability towards the three varieties bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis), while the four complexes failed to be suggested in treatment of ulcers that caused by Streptococcus facials. So, the prepared compounds could be good alternatives to the common drugs which are used in treatment of ulcers.
<Keywords: β-Lactam; Schiff base; 6-Amino penicillanic; Antibacterial; Antibiotic
Developments in the field of coordination chemistry, which is closely bound up with syntheses and development organic and biological molecules as chelating ligand [1,2]. These molecules have been very extensive in recent years. A study of biomolecules ligand complex formation is interested to medical and pharmaceutical fields, because of its high biological activity against bacteria, fungi and tumors, in addition to its high stability as coordination compounds and solubility in the general solvents [3].
Schiff bases ligands have often been used as chelating ligands in the field of coordination chemistry. Chelating ligands containing N, S and O donor atoms show broad biological activity and are of special interest because of the variety of ways in which they are bonded to metal ions. These ligands can be classified by the number of site that gives electrons to the ion metallic (single claw Bidentate, tridentate, Tetradentate, etc.,) [4-9], it is known that the existence of metal ion bonded to biologically active compounds may enhance their activities Schiff base ligands with nitrogen donor atoms of the azomethine group and anther donor atoms of the Schiff base ligand such as oxygen of carboxylic group afforded stable complexes with various transition metals. Beta-Lactam considered important item of antibiotics that contain a (C=O) group in β- Lactam ring in structural [10]. 6-Amino Penicillinic acid is considered as important compound in the varieties of β- Lactam types and traded in the first of antibiotics [11].
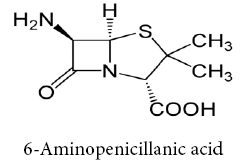
Chemical
All chemicals used were reagent grade (Sigma Aldrich, B.D.H, C.D.H. and S.L. company) including 6-Amino Penicillinic acid, Salicyldehyde, CuCl2.2H2O, CoCl2.6H2O, NiCl2.6H2O, ZnCl2, absolute ethanol, Pyridine, and DMSO.
Instrumentation
The proton 1H and carbon 13C Nuclear Magnetic Resonance (NMR) spectra were recorded in DMSO were recorded on a Bruker Advance II 400 Spectrometer at room temperature the chemical shifts are reported in ppm relative to TMS (tri methyl saline). FT-IR spectra were recorded on 8400 FTIR Shimadzu spectrometer in DMSO-d6 solvent. UVVisible spectra were recorded on Shimadzu 1800 series spectrometer for ligand and complexes in DMSO-d6 solvent. The Magnetic Moment Measurement was using Auto Magnetic Susceptibility Balance Sherwood scientific, The Molar Conductance Measurement in DMSO solvent using Digital conductivity meter alpha -800, addation to mass spectrum.
Preparation of Schiff base ligand
Ligand was prepared by dissolving 6-Aminopenicillanic acid (2.16 g, 0.01 mole) in 15 ml absolute ethanol (basic medium) and then add (1.22 g, 0.01 mole) of Salicyldehyde the result mixture was refluxed for 14 hrs, following up its reaction using thin layer chromatograph, after filtered, dried and returned recrystallized from absolute ethanol the reaction follows illustrates this.
Preparation of complexes
For the preparation of complexes, a ligand (0.700 g, 0.002 mole) solution was mixed with 0.001 mole of (0.170 g, 0.2379 g, 0.2377 g, and 0.136 g) of CuCl2.2H2O, CoCl2.6H2O, NiCl2.6H2O, and ZnCl2 respectively in 15 ml of absolute ethanol. The mixture was refluxed for 4 h on a water bath, then cooling to room temperature, the colored complexes got precipitated slowly, which was filtered, washed repeatedly with distilled water and ethanol. Now, the complexes were dried over anhydrous calcium chloride in desiccators. Table 1 show chemical and physical properties of free ligand and its complexes.
| Compound Symbol | M. Wt. | MP °C | Color |
|---|---|---|---|
| L | 320 | 136-138 | Brown Orange |
| Cu[L]2 | 703.54 | 143-145 | Light Green |
| Co[L]2 | 698.69 | 127-129 | Green |
| Ni[L]2 | 756.93 | 128-130 | Brown |
| Zn[L]2 | 705.39 | 160-162 | Light Brown |
Table 1: Data ligand and complexes show chemical and physical characterizations.
Cases of study
Using cotton swab, samples were collected from focus of different ulcer types for patients suffered from ulcer as a complication for numerous diseases, so they were treated at Al Sadder Medical City in The Al Najaf Al Ashraf Government, Iraq. The phenotypic diagnosis was done according to the Morphological Characteristics after cultivation of these specimens on the MacConkey Agar, Manetol Salt Agar, Brain Heart Infusion Agar, and Pseudomonas Agar (For fluorescing).
Microscopic examination
In order to classify the isolated bacteria from the ulcer specimens the microscopic examination was done at magnification power ×1000, Gram dye was used to classify the isolated bacteria into positive and negative response to Gram dye.
Biochemical tests
Catalase, Oxidase, Indole, Methyl Red, Simmons (Citrate consumption), and Gelatin Lysis Tests were applied to diagnose the isolated bacteria.
Biological activity
Disk Diffusion Method was applied for evaluation of the inhibitor ability of the original ligand and its complexes in the biological activity of the most active plantation of the isolated bacteria (Staphylococcus aureus, pseudomonas aeruginosa, Streptococcus facials and Proteus mirabilis).
Infrared spectra
The FT-IR of prepared Schiff bases were show absorption band at 3259 cm-1 due to υ (OH) of carboxylate group this band shifted to lower frequency in the spectra of all complexes, the bands at 1629 cm-1 and 1720 cm-1 were assigned to stretching vibration of υ(- CH=N-) azomethene and υ(C=O) lactam groups respectively on the complexation these bands have been shifted to higher frequency in the spectra of all complexes. The spectra of complexes recorded a new band in the range of 594-440 cm-1 for coordination with metal, the coordination occurred through the nitrogen atom of azomethene and two oxygen atom of (C=O) lactam and (OH) of carboxylate group. The IR frequencies of free ligand and complexes are reported in Table 2. Figures 1 and 2 show a spectra of free ligand and copper complex [12,13].
| Compound | ν(OH) | ν(C-H)alph. | ν(C-H)alph. | ν(C=O) Lactam | (C=N(Azomethene | M-N M-O |
|---|---|---|---|---|---|---|
| L | 3259 | 2974 | 3064 | 1720 | 1629 | - |
| Cu[L]2 | 3190 | 2976 | 3064 | 1726 | 1635 | 588 451 |
| Co[L]2 | 3232 | 2974 | 3070 | 1728 | 1635 | 580 449 |
| Ni[L]2 | 3236 | 2976 | 3064 | 1726 | 1631 | 594 440 |
| Zn[L]2 | 3379 | 2974 | 3070 | 1728 | 1635 | 532 45 |
Table 2: FTIR data of schiff base ligand and metal complexes.
Electronic spectra
The electronic spectrum of the ligand and complexes have been measured in DMSO solution between 200-1100 nm at room temperature. The UV-Visible spectra of ligand include absorption peaks at wave lengths 288 nm, 270 nm which are assigned to π-π* transition of aromatic ring and n-π* transition for nonbonding pair of electron of nitrogen azomethene group, respectively. In the UV-Vis spectrum of complexes exhibits two peaks, due to the (C.T) [14-16]. The UV-Vis data of ligand and complexes are reported in Table 3 and Uv-Vis spectrum of the Schiff base ligand (Figure 3).
| Complexes | Absorption Bandλmax | Assignment |
|---|---|---|
| L | 2,88,270 | n-π*, π-π * |
| Cu[L]2 | 3,78,287 | C.T |
| Co[L]2 | 429 | C.T |
| Ni[L]2 | 409 | C.T |
| Zn[L]2 | 4,66,424 | C.T |
Table 3: Electronic spectra data of Schiff base ligand and metal complexes.
Mass spectrum
The mass spectrum of free ligand is shown in Figure 4. The spectrum manifestation a peak represents the parent ion (p+1) centered at 307. The general pattern of fragmentation for Schiff base ligand are summarized in Scheme 1 including four proposed pathways for breaking ligand [17,18].
1H-NMR and 13C-NMR spectra of the schiff base ligand
1HNMR spectrum of Schiff base ligand in DMSO-d6 appearance of different peaks (Figure 5). A signal at (δ 8.6) ppm was assigned for singlet due one proton of -CH=N linkage in the ligand. The signal obtained in range (δ 7.2-7.8), other signals of 1H-NMR for ligand have been explained in Table 4. The 13CNMR spectrum of Schiff base ligand also recorded in DMSO-d6 (Figure 6 and Table 5). The 13CNMR spectrum of ligand showed the azomethene carbon peak at (δ 153) ppm. The carboxylic (C=O) group peak showed at (δ 167) ppm and the aromatic carbon peak are observed at (δ 124, 130, 124, 130, 116, 168) ppm [19,20].
| Compound | 1HNMR |
|---|---|
| L | CH3(1.5ppm,S, 6H) |
| Aromatic (7.2-7.8 ppm, m, 5H) | |
| Azomethene (8.6 ppm, S, H) | |
| CHN (4.1 ppm, d, H) | |
| CHCO (4.3 ppm, d, H) | |
| DMSO (3.5,2.5 ppm) |
Table 4: Proton nuclear magnetic resonance spectra of the schiff base ligand.
| Compound | 13C-NMR |
|---|---|
| L | (aromatic) 124,130,124,130,116,168 |
| (azomethene) 153 | |
| (COOH) 167 | |
| (CH3)25 | |
| (CH)79 | |
| (β-Lactam) 80 | |
| (CH)64 | |
| (CHN)76 | |
| (CHCO)62 |
Table 5: Carbon nuclear magnetic resonance spectra of the schiff base ligand.
Magnetic susceptibility and molar conductivity for coordination complexes
Magnetic Susceptibility properties of all complexes measure and compare them with the literature [21,22], which has been reached to form the vacum of these complexes was found to be of the shape octahedral as for molar connectivity every complexes recipe is ionic (neutral) and Table 6 illustrates the magnetic susceptibility and molar conductivity results for the complexes and ligand [23-25].
| Complexes | Magnetic moment | Molar Conductivity | Geometry |
|---|---|---|---|
| Cu[L]2 | 1.7 | 5.9 | Octahedral |
| Co[L]2 | 4 | 8.2 | Octahedral |
| Ni[L]2 | 3.01 | 8.5 | Octahedral |
| Zn[L]2 | Diamagnetic | 5.8 | Octahedral |
Table 6: Magnetic moments and molar conductivity data of schiff base ligand and metal complexes.
Anti-bacterial activity
The prepared complexes of ligand with Cu, Co, Ni and Zn were studied against biological activity of four different types of bacteria (Staphylococcus aureus, Streptococcus facials, Pseudomonas aeruginosa and Proteus mirabilis), at the concentrations of 1 × 10-6, 1 × 10-5, 1 × 10- 4, 1 × 10-3 M and compare to the inhibitory effect of the original ligand (L2) toward same types of bacteria. These bacteria were isolated from the most active plantation of numerous type of ulcer samples (grades III and II burn ulcers, diabetes foot ulcer, in addition to bed ulcers).
The four complexes recorded an elvation in the inhibion ability comparsion to the original ligad which was precarcor of these complexes at different studied concentrations when the ligand and its complexes were applied on the plantation of Staphylococcus aureus as shown in Figure 7 and Table 7.
| Concentration (M) | Compound Symbol | ||||
|---|---|---|---|---|---|
| L | L (1) | L (2) | L (3) | L (4) | |
| 1×10-6 | - | - | - | - | - |
| 1×10-5 | - | + | + | + | - |
| 1×10-4 | - | ++ | + | ++ | - |
| 1×10-3 | + | +++ | +++ | +++ | +++ |
Table 7: Vital effect of ligand land complexes in vital work of bacterial Staphylococcus aureus.
The complex of copper illustrated the highest inhibitory effect among the four complexes, on the other hand the complex of zinc showed approximate inhibitory effectes at the concentration of 1 × 10-6 to 1 × 10-4M, then the ability of inhibition was increased at the greatest tested concentration (1 × 10-3 M), results of the inhibition ability of the cobalt and nickel complexes were so convergent at the four examined concentrations (Figure 7).
Figure 7 illustrates that the four complexes were failed to recored an inhibitory data exceed those which recorded by original ligand even at the greatest tested concentration (1 × 10-3 M) when they applied on the plantation of Streptococcus facials (Table 8 shows the details information about the biological activity of Streptococcus facials at the original ligand and the four prepared complexes had been added).
| Concentration (M) | Compound Symbol | ||||
|---|---|---|---|---|---|
| L | L (1) | L (2) | L (3) | L (4) | |
| 1×10-6 | - | - | - | - | - |
| 1×10-5 | + | - | - | - | - |
| 1×10-4 | ++ | + | - | - | - |
| 1×10-3 | +++ | + | + | + | + |
Table 8: Vital effect of the ligand and complexes in the biological activity of Streptococcus facials.
The first concentration of the prepared complexes couldn’t show significant elevation in the inhibition zone comparison to those at their corresponding ligand, as observed in the Figure 7, then; the ability of inhibition for the complexes towered plantation of Pseudomonas aeruginosa was grgually increased simultaneously with the increase of the complexes̓ concentrations (Table 9 and Figure 7).
| Concentration (M) | Compound Symbol | ||||
|---|---|---|---|---|---|
| L | L(1) | L(2) | L(3) | L(4) | |
| 1×10-6 | - | - | - | - | - |
| 1×10-5 | - | + | - | - | - |
| 1×10-4 | - | ++ | ++ | ++ | ++ |
| 1×10-3 | + | +++ | +++ | +++ | +++ |
Table 9: Vital effect of the ligand and complexes in the biological activity of Pseudomonas aeruginosa.
Figure 7 exposes the ability of inhibition data for the ligand and its complexes at they applied in the plantation of Proteus mirabilis, the complex of cobalt in addition to the original ligand were illustrated the highest inhibitory effect followed by the complexes of nickel then zinc, respectevily, while complex of copper demonstrated approximate inhibitory effects at different concentrations, as shown in Table 10 and Figure 7.
| Concentration (M) | Compound Symbol | ||||
|---|---|---|---|---|---|
| L | L(1) | L(2) | L(3) | L(4) | |
| 1×10-6 | + | ++ | + | + | + |
| 1×10-5 | ++ | + | ++ | + | + |
| 1×10-4 | +++ | ++ | ++ | ++ | + |
| 1×10-3 | +++ | ++ | +++ | +++ | +++ |
Table 10: Vital effect of the ligand and complexes in the biological activity of Proteus mirabilis.
The prepared ligand and it’s complexes illustrated a good inhibitory ability towards the three varieties bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis), while the four complexes failed to be suggested in treatment of ulcers that caused by Streptococcus facials. So, the prepared compounds could be good alternatives to the common drugs which are used in treatment of ulcers.