Journal of Theoretical & Computational Science
Open Access
ISSN: 2376-130X
ISSN: 2376-130X
Research Article - (2015) Volume 2, Issue 2
In this work, Indium oxide (In2O3) thin film is successfully deposited on microscopic glass substrate at different temperatures by spray pyrolysis technique using InCl3 as precursor. The physical properties of these films are characterized by XRD, SEM, AFM, FT-IR, FT-Raman, UV-visible and AFM measurements. XRD analysis exposed that the structural transformation of films from stoichiometric to non-stoichiometric orientation of the plane vice versa and also found that, the film is polycrystalline in nature having cubic crystal structure with a preferred grain orientation along (222) plane. SEM and AFM studies revealed that, the film with 0.1M at 500â°C has spherical grains with uniform dimension. The complete vibrational analysis has been carried out and the optimized parameters are calculated using HF and DFT (CAM-B3LYP, B3LYP and B3PW91) methods with 3-21G (d,p) basis set. Furthermore, NMR chemical shifts are calculated by using the gauge independent atomic orbital (GIAO) technique. The molecular electronic properties; absorption wavelengths, excitation energy, dipole moment and frontier molecular orbital energies, molecular electrostatic potential energy (MEP) analysis and Polarizability first order hyperpolarizability calculations are performed by time dependent DFT (TD-DFT) approach. The energy excitation on electronic structure was investigated and the assignment of the absorption bands in the electronic spectra of steady compound is discussed. The calculated HOMO and LUMO energies showed the enhancement of energy gap by the addition of substitutions with the base molecule. The thermodynamic properties (heat capacity, entropy, and enthalpy) at different temperatures are calculated and interpreted in gas phase.
<Keywords: Indium Oxide, Spherical grains, Gauge independent atomic orbital, MEP, Frontier molecular orbital energy, Hyperpolarizability
Indium oxide (In2O3) is a significant and a well-recognized translucent conducting oxide of intrinsic semiconductor exhibiting a narrow band gap. So it has considerable chemical stability, high electrical conductivity and elevated optical activity. It is commonly used for preparation of photovoltaic devices, transparent windows; liquid crystal displays (LCD), light emitting diode (LED), solar cell, gas sensors and anti- reflecting coatings [1]. Indium oxide (In2O3) thin films with their non-stoichiometric form having wide band gap (Eg ~ 3.7 eV [2]) are very important materials for solar energy conversion and gas sensing applications due to their high transparency in the visible and near infrared regions, low resistivity and good adherence to substrates [3-5]. In2O3 is an amphoteric oxide of indium, n-type semi-conductor, which crystallizes within a bixbyite-type cubic crystal structure and it is known to have a body centred cubic structure (a=10.11 Å) and with Ia3 space group [6]. Among the various transparent conducting oxides, In2O3 has attracted tremendous attention due to its enormous applications in many fields. It is used in thin film infrared reflectors transparent for visible light (hot mirrors), electroluminescent devices, antireflection coatings [7], photocatalyst sensors, electro chromic devices [4], photo thermal devices [8], light-emitting diodes [9], some types of batteries, resistive elements and integrated circuits etc. It can also be used in optoelectronic devices, thin film solar cells [10] and gas sensors [11].
In2O3 films have been deposited using numerous physical and chemical methods such as reactive evaporation [12], pulsed laser evaporation [13], sputtering [14], sol–gel technique [15], chemical vapour deposition [16] and spray pyrolysis [17]. Among these methods, spray pyrolysis technique has many advantages such as low cost of the apparatus and source materials, control over the deposition parameters and producing large area films deposition compared to other deposition methods. In2O3 films were deposited so far using InCl3, InNO3, Indium acetate and In-acac precursors. Among these precursors, InCl3 is a predominant source material for In2O3 films with favourable physical properties.
In the present work, the effects of substrate temperature and precursor concentration on sprayed In2O3 films using InCl3 precursor have been studied. The important characterizations; XRD, SEM with EDAX of deposited film with different molarities at a constant substrate temperature was studied and the optimum molarity was fixed. Then the optimum temperature was found by various studies of deposited In2O3 films at various substrate temperatures with this optimum molarity. The scope of this work is to optimize the molar concentration of InCl3 precursor and substrate temperature in order to obtain highly transparent conductive In2O3 films.
In2O3 films were deposited with different molarities and various temperatures by a pneumatic controlled spray pyrolysis. InCl3 and de ionized water mixtures sprayed on to glass substrates with dimension of 75 x 25 mm2. The precursor concentration was varied from 0.05 to 0.15M by keeping substrate temperature and other deposition parameters as constant. Then the substrate temperature was varied from 350 to 550°C by keeping precursor concentration and other deposition parameters as constant. In the spray pyrolysis unit, the substrate temperature was maintained with the help of a heater and an electronic circuitry, which contains a thermal sensor with relay switch. The precursor solution and career gas assembly connected to spray gun was moved in the horizontal plane by means of a pneumatic controlled system. The substrate to nozzle distance was maintained at 25 cm with angle of 45°. The films deposited on pre-cleaned glass plates were gradually cooled to room temperature and then rinsed with de ionized water and dried.
The well-adherent In2O3 films underwent structural, morphological and optical studies. The structural studies were carried out by using on SHIMADZU-6000 X-ray diffractometer (XRD) equipped with Cu-Kα radiation (λ=1.5418Å). The surface morphological studies were examined by scanning electron microscope (SEM) HITACHI S-3400N and atomic force microscope (AFM) NANONICS MV 1000. The optical absorption and photoluminescence spectra were recorded using JASCOV-670 spectrophotometer and Plorolog3-HORIBA JOBIN-YVON, respectively.
The prepared compound In2O3 is used for recording the spectra as such without any further purification. The FT-IR spectrum of the compound is recorded in Bruker IFS 66V spectrometer in the range of 1000–50 cm−1. The spectral resolution is ±2 cm−1. The FT-Raman spectrum of same compound is also recorded in the same instrument with FRA 106 Raman module equipped with Nd:YAG laser source operating at 1.064 μm line widths with 200 mW power. The spectra are recorded in the range of 1000–50 cm−1 with scanning speed of 30 cm−1 min−1 of spectral width 2 cm−1. The frequencies of all sharp bands are accurate to ±1 cm−1.
In the present work, HF and DFT calculations have been performed on In2O3 to inspect the vibrational behaviour and electronic structure properties. This tremendous time consuming higher level larger calculations has been executed using Gaussian09 package on Core i3 processor in personal computer. The computed results were visualized by Gauss View05 [18,19] graphical user interface to produce spectra and vivid molecular plots etc. HF and some of the hybrid methods of DFT; B3LYP and B3PW91 were carried out using the basis set 3-21G(d,p). In DFT methods; Becke’s three parameter hybrids function combined with the Lee-Yang-Parr correlation function (B3LYP) [20,21], Becke’s three parameter exact exchange-function (B3) [22] combined with gradient-corrected correlational functional of Lee, Yang and Parr (LYP) [23,24] and Perdew and Wang (PW91) [24] predict the best results for molecular geometry and vibrational frequencies for moderately larger molecules. The calculated frequencies are scaled down to yield the coherent with the observed frequencies. The scaling factors are 0.621, 0.860, 0.790, 0.775, 0.827 and 0.960 for HF and DFT methods.
The optimized molecular structure of the molecule is obtained from Gaussian 09 and Gauss view program Figure 1. The comparative optimized structural parameters such as bond length, bond angle and dihedral angle are presented in Table 1. The observed (FT-IR and FTRaman) and calculated at HF and DFT (B3LYP and B3PW91) with 3-21G(d,p) basis set vibrational frequencies, vibrational assignments of present molecule are presented in Table 2. Experimental and simulated spectra of IR and Raman are presented in the Figures 2 and 3 respectively.
| Geometrical Parameters | Methods | Experimental value | |||
|---|---|---|---|---|---|
| HF/3-21G(d,p) | CAM-B3LYP/3-21G(d,p) | B3LYP/3-21G(d,p) | B3PW91/3-21G(d,p) | ||
| Bond length(Â) | |||||
| In1-O2 | 1.875 | 1.857 | 1.875 | 1.867 | 1.81 |
| In1-O3 | 1.905 | 1.941 | 1.967 | 1.959 | 1.85 |
| O3-In4 | 1.905 | 1.941 | 1.967 | 1.959 | 1.85 |
| In4-O5 | 1.875 | 1.857 | 1.875 | 1.867 | 1.81 |
| Bond angle(°) | |||||
| In1-O3-In4 | 129.99 | 129.4 | 125.9 | 125.88 | 128.0 |
| O2-In1-O3-In4 | 180.0 | 180.4 | 181.6 | 181.99 | 180.0 |
| O3-In4-O5-In1 | 180.0 | 180.4 | 181.6 | 181.99 | - |
| O2-In1-O3-In4 | 180.0 | 180.0 | 180.0 | 180.0 | - |
| O3-In4-O5-In1 | 180.0 | 180.0 | 180.0 | 180.0 | - |
Table 1: Optimized geometrical parameters for Indium oxide computed at HF and DFT [CAM-B3LYP, B3LYP and B3PW91] methods with 3-21G(d,p) basis sets
| S. No. | Observed Frequency(cm-1) | Calculated frequency (cm-1) [scaled] | Vibrational assignments | Species | ||||
|---|---|---|---|---|---|---|---|---|
| HF 3-21G (d,p) | CAM-B3LYP 3-21G (d,p) | B3LYP 3-21G (d,p) | B3PW91 3-21G (d,p) | |||||
| FT-IR | FT-Raman | |||||||
| 1 | 600s | - | 690 | 610 | 608 | 606 | (In-O)υ Asym | B2 |
| 2 | 580m | 580s | 610 | 585 | 592 | 593 | (In-O)υ Asym | B2 |
| 3 | 490s | - | 520 | 498 | 496 | 498 | (In-O)υ Sym | A1 |
| 4 | 390m | 390w | 394 | 400 | 395 | 402 | (In-O)υ Sym | A1 |
| 5 6 | 300m | 300m | 318 | 314 | 312 | 314 | (In-O) δ | A1 |
| 6 | 260m | 260w | 267 | 268 | 258 | 271 | (In-O-In) δ | A1 |
| 7 | 210m | - | 224 | 225 | 120 | 110 | (In-O-In) δ | A1 |
| 8 | 200w | 200w | 90 | 78 | 90 | 88 | (In-O-In) γ | A2 |
| 9 | 110w | - | 60 | 50 | 78 | 78 | (In-O) γ | B1 |
| Unscaled values | ||||||||
| 1 | 1062 | 897 | 844 | 857 | ||||
| 2 | 745 | 827 | 793 | 807 | ||||
| 3 | 725 | 802 | 762 | 775 | ||||
| 4 | 253 | 424 | 430 | 435 | ||||
| 5 | 149 | 126 | 128 | 127 | ||||
| 6 | 128 | 97 | 102 | 100 | ||||
| 7 | 74 | 96 | 82 | 87 | ||||
| 8 | 74 | 71 | 70 | 70 | ||||
| 9 | 47 | 60 | 68 | 65 | ||||
vs; very strong, s; strong, m; medium, w; weak, vw; very weak. υ; stretching, δ; in plane bending: γ; out of plane bending:
Table 2: Observed and calculated vibrational frequencies at HF & DFT (CAM-B3LYP/B3LYP/ B3PW91) with 3-21G (d,p) basis set of In2O3
The NMR isotropic shielding are calculated with the GIAO method [24] using the optimized parameters obtained from B3LYP/3-21G(d,p) method. The isotropic chemical shifts at B3LYP methods with 3-21G(d,p) level using the IEFPCM method in DMSO and methanol. The electronic properties; HOMO-LUMO energies, absorption wavelengths and oscillator strengths are calculated using B3LYP method of the time-dependent DFT (TD-DFT) [25,26], basing on the optimized structure in gas phase and solvent[DMSO and methanol] mixed phase. Thermodynamic properties have been calculated between 100-1000°C in gas phase using B3LYP/3-21G(d,p) method. Moreover, the dipole moment, nonlinear optical (NLO) properties, linear polarizabilities and first hyperpolarizabilities and chemical hardness have also been studied.
Molecular geometry
In2O3 compound possess a BCC molecular structure and belong to C2V point group symmetry. The most stable V-shape structure is optimized at (222) plane which is confirmed by other analysis. The zero point vibrational energy of the compound in HF, CAM-B3LYP, B3LYP and B3PW91 are 4.66, 4.69, 4.75 and 4.86 KCal/mol, respectively. The present molecule has bixbyite type-structure which is composed of In-O bonds symmetrically. In the molecule, O is centred and two In and O atoms coupled symmetrically with equal internuclear distance. The In and O atoms are coupled linearly and form the chain structure. The experimental value of In-O bond length in the chain is 1.81 and 1.85Å respectively, [27,28] whereas the calculated In-O bond lengths in the chain are 1.87 to 1.96 Å respectively. From bond length, it is observed that, the unequal distribution of internuclear forces between oxygen and indium atoms making the bond distance different that form the hexagonal plane to attain V shape structure.These bond lengths are well disturbed by the temperature applied on the substrate and thereby the structure is what disturbed over 550°C. The calculated bond angle of In-O-In is 129° in the chain whereas the experimental value is 128°. Similarly, the calculated bond angle O2-In1-O3-In4 is 180.4° whereas its experimental value is 180° [28]. The entire observed crystal data are greater than the calculated values. The precise V-shape rhombus like C2V point group molecular structure is obtained by acquisition of high coordination number.
Structural Studies
Effect of molar concentration
The XRD patterns of In2O3 films deposited at various molar concentrations Figure 4 exhibit polycrystalline nature corresponding to a body centered cubic (BCC) structure with Ia3 space group. The peak positions are in good agreement with JCPDS card No-06-0416. The calculated lattice constant for all films, a=10.05Å, is slightly less than the standard value 10.11 Å [6]. This slight variation of unit cell dimension is due to tight packing of In2O3 cubic crystal. In the film deposited at 0.05M, only (222) and (400) planes are present. With increasing molarity to 0.1M, the intensity of (400) plane is reduced and (211), (440) and (622) planes appeared but with very low intensity. Further increasing molarity to 0.15M, (400) plane intensity is increased drastically. Therefore, it has been concluded that 0.1M film has (222) plane with a the highest intensity exhibits improved crystallinity and the best degree of film texturing; I(222)/I(400) ratio is observed to be maximum.
From X-ray diffraction (XRD) pattern, the micro structural and structural parameters can be estimated from the broadening of the most intense (222) reflection; including (i) the grain size from Scherer- Bragg equation (equation1); (ii) the micro strain (ε) (equation 2); (iii) the dislocation density (δ) (equation 3); (iv) the inter planar spacing (dhkl) (equation 4):
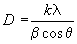 (1)
(1)
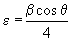 (2)
(2)
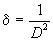 (3)
(3)
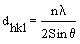 (4)
(4)
The calculated parameters are listed in Table 3. It shows that the 0.1M has favourable structural and micro structural parameters rather than other molarities, with lowest micro strain (0.76 x 10-3) and dislocations density (4.4 x 1014 m-2) and average particles size around 48 nm.
| Molar concentration | Grain size (D) (nm) | Micro strain ε x10-3 | Dislocation Density x1014 (m-2) | Inter planar spacing (nm) |
|---|---|---|---|---|
| 0.05 M | 27 | 1.05 | 13.8 | 0.266 |
| 0.1 M | 48 | 0.76 | 4.4 | 0.262 |
| 0.15 M | 27 | 1.32 | 13.3 | 0.245 |
Table 3: Grain size, Micro strain, Dislocation density and Inter-planar spacing of In2O3 films deposited at different molarities
Effect of temperature
XRD spectra of In2O3 films deposited at different substrate temperatures from 350 to 550°C at the optimized molarity of 0.1M are shown in Figure 4. All the films are polycrystalline in nature and are in good agreement with the standards as discussed earlier in Figure 5. For the film deposited at 350°C the plane (400) is present with a higher intensity than the (222) plane, suggesting a preferred orientation along (400) reflection. In the temperature range 400-500°C, the intensity of (222) plane increases while that of (400) decreases, thereby a new preferred orientation raises along (222) reflection. From the result, it is inferred that the structural transformation from stoichiometric to non-stoichiometric form of In2O3 crystal. Then, at 550°C, the intensity of (400) plane again increases due to the structural re-orientation of grains. The relative (222)/(400) intensity ratio changes suggesting that there is no particular preferred orientation. Thus, the film deposited at 500°C revealed improved crystallinity and maximum degrees of film texturing.
The micro structural parameters estimated from (222) plane for different substrate temperatures calculated using the above mentioned relations are listed in Table 4. It shows that In2O3 film deposited for 0.1M at 500°C has the highest values of grains size (48 nm) and interplanar spacing (0.262 nm) with minimum micro strain (0.76 x 10-3) and dislocation density (4.4 x 1014 m-2). Accordingly the film for 0.1M with a substrate temperature of 500°C has the most favourable structural and micro structural parameters rather than the other molarities and temperatures.
| Temperature (in°C) | Grain size D(nm) | Micro strain ε x10-3 | Dislocation Density δ x1014(m-2) | Inter planar spacing(nm) |
|---|---|---|---|---|
| 350 | 35.901 | 1.01 | 7.758 | 0.250 |
| 400 | 34.584 | 1.05 | 8.360 | 0.244 |
| 450 | 47.167 | 0.77 | 4.494 | 0.258 |
| 500 | 47.750 | 0.76 | 4.385 | 0.262 |
| 550 | 36.324 | 1.0 | 7.578 | 0.244 |
Table 4: Grain size, Micro strain, Dislocation density, Inter-planar spacing of In2O3 films deposited at different temperatures
Morphological studies
SEM images of films Figure 6 at different substrate temperatures for the optimized molarity 0.1M. It is evident that the grains size change with temperature is in close agreement with XRD results. From SEM, it is observed that, the film for 0.1M at 500°C having spherical grains with almost uniform dimensions. Figure 7 EDS of In2O3 thin film deposited at 500°C. The atomic ratio of In and O elements of the present compound is 23.5 and 76.5 respectively. Figure 8 AFM images, where the smooth and uniform surface morphology is formed by spherical particles with homogeneous size distribution around 50 nm. This is supported by the observations obtained by SEM analysis in the past report. The estimated average roughness value is found to be 9.6 nm which is a confirmation of the deposition of smooth In2O3 film.
Optical Properties of In2O3 Films
The optical transmittance spectra Figure 9 of In2O3 films deposited at different substrate temperatures. It can be noticed that the film deposited at 350°C exhibits different optical behaviour rather than the films deposited at higher temperatures. From such a result, it inferred that, the film deposited at 350°C is in stoichiometric form and above 350°C; the structural transformation is occurred from stoichiometric to non- stoichiometric. Furthermore, with increasing substrate temperature, the percentage of transmittance increases with increasing of wavelength up to 450°C. The films deposited within the temperatures range 450-500°C having maximum transmittance percentage. At 500°C, the perfect interference pattern can be observed which is due to structural homogeneity and uncluttered surface morphology.
The optical band gap of the deposited In2O3 films is evaluated from the relation between absorption coefficient α and photon energy hν [29]:
(αhν) = A (hν-Eg)x (5)
where A is a constant, Eg is the optical band gap and x =1/2 for directly allowed electronic transitions. Figure 10 shows the plot between (αhν)2 and hν of the deposited films at 350-550°C.The extrapolation of linear portion of the curves along hν axis gives the direct band gap energy. The band gap increases from 3.062 eV to 3.702 eV with increasing the substrate temperature (Table 5), which is attributed to the increased carrier density due to Brestein-Moss effect. This effect is due to the filling of states near the bottom of the lowest state in the conduction band [30].
| Temperature (in°C) | Band gap energy (eV) | Refractive index | Extinction coefficient(min) | Dielectric constant Real part | Dielectric constant imaginary part | PL intensity x 106 (a.u) |
|---|---|---|---|---|---|---|
| 350 | 3.062 | 2.60 | 0.045 | 7.02 | 0.291 | 0.30 |
| 400 | 3.598 | 2.63 | 0.005 | 6.98 | 0.179 | 0.22 |
| 450 | 3.680 | 2.64 | 0.002 | 6.95 | 0.171 | 1.46 |
| 500 | 3.661 | 2.68 | 0.003 | 7.19 | 0.153 | 6.46 |
| 550 | 3.702 | 2.64 | 0.004 | 6.95 | 0.129 | 1.43 |
Table 5: Optical parameters of In2O3 films deposited at different temperatures
The extinction coefficient (k) can be determined using the relation [31]:
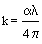 (6)
(6)
Figure 11 shows the variation of the extinction coefficient with wavelength for different substrate temperatures. It is indicated that the extinction coefficient almost decreases with increasing of temperature. It may be due to the increase of adhesivity of the films with increasing substrate temperature. The value of the extinction coefficient of the films deposited at 450 and 500°C is very low, meanwhile at 500°C, it exhibits better variation of k between U-V and visible regions than the other temperatures.
The refractive index (n) of the film can be evaluated using the relation [32]:
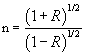 (7)
(7)
where R is the normal reflectance. Figure 12 shows the variation of refractive index with wavelength of the films deposited at different temperatures. The spectra of all films revealed that the maximum of refractive index is in the U-V region except the film deposited at 350°C. It deep-rooted again in the XRD discussion as that is the stoichiometric form of In2O3 at 350°C. From the spectra, it is accomplished that, the film deposited for 0.1M at 500°C has the maximum refractive index of 2.68 at 371 nm (Table 5).
The dielectric constant (ε) of the films is determined by the relation [33]:
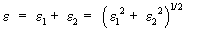 (7)
(7)
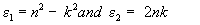 (8)
(8)
where, ε1 and ε2 are the real and imaginary parts of the dielectric constant
The dielectric constant or the absolute complex permittivity of a material depends on temperature, pressure and frequency. Figure 13 is the frequency dependence of the dielectric constant for the films deposited at different temperatures. From the figure, it is clear that, the frequency dependence of dielectric constant is found to vary with substrate temperature, which may be due to the electronic polarization of the material.
The photoluminescence spectra of In2O3 films are obtained using bombardment of an excitation source with a wavelength of 325 nm. Figure 14 shows PL spectra of the films deposited at different substrate temperatures. Only one emission peak in the violet region can be observed for the film deposited at 500°C. Whereas an intense indigo light emission is observed at 441 and 435 nm for the film deposited at temperatures 350 and 400°C respectively. From PL spectra with varying substrate temperature, the intensity of PL emission is observed to be maximum at 500°C, which implies that more radiated recombination occurs with the excitation source of wavelength of 325 nm due to improved crystalline structure. The observed optical parameters of In2O3 films deposited at different molarities and different temperatures are listed in Table 5.
Vibrational assignments
In order to obtain the spectroscopic signature of the In2O3, the computational calculations are carried out for frequency analysis. The molecule is identified with C2V point group symmetry, consists of 5 atoms, so it has 9 normal vibrational modes. On the basis of C2V symmetry, the 9 fundamental vibrations of the molecule can be distributed as
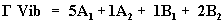 (8)
(8)
A1 and B2 irreducible representations correspond to stretching, ring deformation and in plane bending vibrations while A2 and B1 correspond to ring, torsion and out of plane bending vibrations. The harmonic vibrational frequencies (unscaled and scaled) calculated at different levels using the triple split valence basis set along with the diffuse and polarization functions. Comparison of calculated frequencies with the experimental values reveal the over estimation of the calculated vibrational modes due to the neglect of a harmonicity and change of state of real system.
In-O vibrations
A simple metal oxide coupled with more than one oxygen’s, usually absorbs the radiation by vibrations in the region 1020-970 cm-1 [34]. Because of its heavy mass, the In-O stretching vibrations are found in the region 800-300 cm-1 [35-37]. The In-O bond stretching is usually observed in the region 700-300 cm-1 [38]. In this present case, In2O3 chain consists of few hetero nuclear bonds, the In-O asymmetric and symmetric stretching vibrations found with strong intensity at 600, 580 and 490 and 390 cm-1, respectively. According to the literature, these vibrational modes are observed within the expected region. The In-O in plane bending vibrations appeared at 300, 260 and 210 cm-1. The In-O out of plane bending vibrations appeared at 200 and 110 cm-1. The entire bending vibrations of In-O bonds are identified within the expected region. Hence, the entire vibrational pattern is observed in the low frequency region since the In2O3 is more stable and heavy mass.
NMR examination
Nuclear magnetic resonance (NMR) spectroscopy is a valuable technique for obtaining chemical information of tricky complex molecules. This is because the spectra are very sensitive to changes in the molecular structure. This same sensitivity makes NMR a difficult case for molecular modelling. Computationally predicting coupling constants is much easier than predicting chemical shifts. Because of this, the ability to predict coupling constants is sometimes incorporated into software packages that ability to predict chemical shifts. In this way, the optimized structure of present molecule was used to calculate the NMR spectra at B3LYP method with 3-21G(d,p) level using the GIAO method and the chemical shifts of the compound are reported in ppm which are presented in Table 6.
| Atom position | Gas | DMSO | Chloroform |
|---|---|---|---|
| In1 | 3639 | 3771 | 3737 |
| In2 | 3639 | 3771 | 3737 |
| O2 | 3412 | 2094 | 2418 |
| O3 | 1123 | 739 | 832 |
| O5 | 3412 | 2094 | 2418 |
Table 6: Experimental and calculated NMR chemical shifts (ppm) of In2O3
Normally, the range of NMR chemical shifts for organic compound is greater than 100 ppm [39,40] and the accuracy ensure that the reliable interpretation of spectroscopic parameters. The present molecule is a chain in which the chemical shift of In is 3639, 3771 and 3737 ppm in gas and solvent phase respectively. The entire calculated values of chemical shift are high which is restricted by coupling of O only. The chemical shift of O3 is 1123 ppm and O2 and O5 are 3412. Particularly, the chemical shift of O3 is less than rest of others. Because O3 is symmetrically shielded by In atoms.
The chemical shift of entire atoms in the chain is more since present compound is more stable. The chemical shift of O is controlled by In. From the observation, it is clear that the chemical property of O is altered by the coupled atom In. Thus the dielectric property of oxide and the conducting property of Indium are mixed and the In2O3 gets semiconducting property. In addition to that, there is some difference chemical shift between gas and solvent phases. From the NMR observation, it was clear that, the molecule is fused by two different atoms and thereby the property of the product is tuned new.
Electronic properties (frontier molecular analysis)
The frontier molecular orbitals are very much useful for studying the electric and optical properties of the organic molecules. The stabilization of the bonding molecular orbital and destabilization of the antibonding can be made by the overlapping of molecular orbitals. The stabilization of the bonding molecular orbital and destabilization of the antibonding can increase when the overlap of two orbitals increases [41].
In the molecular interaction, there are the two important orbitals that interact with each other. One is the highest energy occupied molecular orbital is called HOMO represents the ability to donate an electron. The other one is the lowest energy unoccupied molecular orbital is called LUMO as an electron acceptor. These orbitals are sometimes called the frontier orbitals. The interaction between them is much stable and is called filled empty interaction. When the two same sign orbitals overlap to form a molecular orbital, the electron density will occupy at the region between two nuclei. The molecular orbital resulting from in-phase interaction is defined as the bonding orbital which has lower energy than the original atomic orbital. The out of phase interaction forms the anti-bonding molecular orbital with the higher energy than the initial atomic orbital.
The 3D plots of the frontier orbitals, HOMO and LUMO for Indium oxide molecule are in gas, Figure 15. According to Figure 15, the HOMO is mainly localized over the O atoms of the chain. The region of high electron density which hold the three nuclear centres together to form a sigma bond interaction taking place over entire O in the chain. The In atoms are connected by SP hybrid orbital lobes. The antibonding orbital lobes are taking place over In atoms of the molecule. However, LUMO is characterized by a charge distribution connected the O-In-O by making two sigma bonds in the chain. There are two antibonding lobes overlapping taking place over the O. From this observation, it is clear that, the in and out of phase interaction were present in HOMO and LUMO respectively. The HOMO→LUMO transition implies an electron density transferred among O and In in the chain. The HOMO and LUMO energy are 6.570 eV and 3.419 eV in gas phase. Energy difference between HOMO and LUMO orbital is called as energy gap (kubo gap) that is an important stability for structures. The actual calculated energy gap is 3.157 eV, which shows the low energy gap and reflect the high electrical activity of the molecule. So, the thin film material under study acts as a pure semiconductor.
Optical properties (HOMO-LUMO analysis)
The UV and visible spectroscopy is used to study the optical property the molecule and also to detect the optical activity (NLO properties) of the compound. The calculations of the electronic structure of present compound were optimized in singlet state. The low energy electronic excited states of the molecule were calculated at the B3LYP/6-311++G(d,p) level using the TD-DFT approach on the previously optimized ground-state geometry of the molecule. The calculations are performed in gas phase and with the solvent of DMSO and methanol. The calculated excitation energies, oscillator strength (f) and wavelength (λ) and spectral assignments were given in Table 7. The major contributions of the transitions are designated with the aid of SWizard program [42].
| λ (nm) | E (eV) | ( f ) | Major contribution | Assignment | Region | Bands |
|---|---|---|---|---|---|---|
|
Gas |
|
|||||
| 1415.2 |
0.876 |
0.003 | H→L (95%) | n→ σ* | IR-Visible | B-band |
| 1211.3 | 1.023 | 0.00 | H→L (90%) | n→ σ* | ||
| 1193.3 | 1.038 | 0.001 | H→L (88%) | n→ σ* | ||
|
DMSO |
|
|||||
| 1149.1 | 1.078 | 0.004 | H→L (94%) | n→ σ* | IR-Visible | B-band |
| 1014.6 | 1.222 | 0.00 | H→L (92%) | n→ σ* | ||
| 1013.2 | 1.223 | 0.004 | H→L (88%) | n→ σ* | ||
|
Methanol |
|
|||||
| 1151.9 | 1.076 | 0.00 | H→L (94%) | n→ σ* | IR-Visible | B-band |
| 1016.5 | 1.219 | 0.00 | H→L (92%) | n→ σ* | ||
| 1014.6 | 1.222 | 0.004 | H→L (88%) | n→ σ* | ||
Table 7: Theoretical electronic absorption spectra of In2O3 (absorption wavelength λ (nm), excitation energies E (eV) and oscillator strengths (f) using TD-DFT/ B3LYP/3-21G (d,p) method in DMSO, Methanol and gas phase.
TD-DFT calculations predict that, irrespective of the gas and solvent phase, the entire transitions belong to quartz ultraviolet region. In the case of gas phase, the strong transitions are at 1415, 1211 and 1193 nm with an oscillator strength f=0.003, 0.00 and 0.001 with 0.87, 1.02 and 1.03 eV energy gap. The entire transitions were belong to n→ σ* in IR and visible region. The designation of the band is B-band (Benzenoid) which is attributed to above said transition of chain, such as In and O. In this compound, the atom In is attached with auxochrome O and undergo hypsochromic to bathochromic shift. They are characterized by low molar absorptivities (ξmax>1000) and the solvent effect was less in this compound.
In the case of DMSO solvent, strong transitions were 1149, 1014 and 1013 nm with an oscillator strength f=0.004, 0.00 and 0.004 with maximum energy gap 1.223 eV. They also assigned to n → σ* transition and belongs to IR-visible region. As in the Figure 16, the optical energy gap is 2.29 eV which is very low. Whereas according to the UV table it is 1.22 eV. From this observation, it is clear that, the threshold electronic transition starts from 1.2 eV but such the transitions are forbidden. It is also found that, the electronic transitions retained at IR-visible region when from gas to solvent. This view indicates that, the In2O3 thin film is Visible active and it is capable of having rich photo conductive properties. In view of calculated absorption spectra, the maximum absorption wavelength corresponds to the electronic transition from the HOMO to LUMO with maximum contribution.
Molecular Electrostatic Potential (MEP) maps
The electrostatic potential, especially when computed on molecular surfaces, is a powerful tool for analyzing and interpreting reactive behaviour. It is particularly effective for covalent and noncovalent interactions and the early stages of processes that eventually involve bond formation. It can be shown that, the potential has qualitative and quantitative predictive capacity. Because it can be obtained purely computationally, using optimized molecular geometries, the potential can be used to characterize compounds and to design them to have specific desired features [43,44]. With the continuing remarkable developments in methodology, software, and processor technology, the different applications of the electrostatic potential can be expected to increase further in scope and reliability, in medicinal chemistry as in other areas.
Molecular electrostatic potential view is mapped up with optimized geometry at the level of B3LYP/3-21G(d,p) theory. There is a great deal of intermediary potential energy, the non-red or blue region indicate that the electro negativity difference is not very great. In a molecule with a great electro negativity difference, charge was very polarized in negative and positive form, and there are significant differences in electron density in different regions of the molecule. This great electro negativity difference leads to regions that are almost entirely red and almost entirely blue. The region of intermediary potential is explored by yellow and green colour and the regions of extreme potential look at red and blue colours are key indicators of electronegativity.
The colour code of these maps is in the range between -3.25 a.u. (deepest red) to 3.25 a.u. (deepest blue) in compound. The positive (blue) regions of MEP are related to electrophilic reactivity and the negative (green) regions to nucleophilic reactivity shown in Figure 17. As can be seen from the MEP map of the title molecule, the negative regions are mainly localized over the portion where the O atoms present. A maximum positive region is localized over bending portion (at O) of a molecule indicating a possible site for nucleophilic attack. From these results, it is inferred that, when this molecule coupled with other compound, it will bind strongly and such a part act as main root to bind.
Polarizability and higher order polarizability analysis
The polarizabilities and first order hyperpolarizabilities determine the dynamical response of the bound molecule to the external fields and provide insight into a molecule’s internal structure. In order to investigate the relationships among the molecular structures, nonlinear optic properties (NLO) and molecular binding properties, the polarizabilities and first order hyperpolarizabilities of the present compound were calculated using DFT-B3LYP method and 3-21G(d,p) basis set, based on the finite-field approach.
The Polarizability and hyperpolarizability tensors (αxx, αxy, αyy, αxz, αyz, αzz and βxxx, βxxy, βxyy, βyyy, βxxz, βxyz, βyyz, βxzz, βyzz, βzzz) is obtained from the output file of Polarizability and hyperpolarizability calculations. However, α and β values of Gaussian output are in atomic units (a.u.) have been converted into electronic units (esu) (α; 1 a.u. = 0.1482×10−24 esu, β; 1 a.u. = 8.6393×10−33 esu).
In Table 9, the calculated parameters described above and electronic dipole moment {μi (i = x, y, z) and total dipole moment μtot} for title compound are listed. It is well known that, molecule with high values of dipole moment, molecular Polarizability, and first hyperpolarizability having more active NLO properties. The first hyperpolarizability (β) and the component of hyperpolarizability βx, βy and βz of In2O3 along with related properties (μ0, αtotal, and Δα) are reported in Table 8. The calculated value of dipole moment is found to be 2.098 Debye. The highest value of dipole moment is observed in the component of μz which is 2.098 D. The lowest value of the dipole moment of the molecule compound is μx & μy component (0.0 D). The calculated average Polarizability and anisotropy of the Polarizability is 55.47x10-24 esu and 49.23x10−24 esu, respectively. The magnitude of the molecular hyperpolarizability β, is one of important key factor in a NLO system. The calculated first order of hyperpolarizability value (β) is 115.04x10−30 esu by B3LYP/3-21G(d,p). From the above results, it is observed that, the molecular Polarizability and hyperpolarizability of the title compound are active. So that, the present compound can be used to prepare NLO crystals and these crystals is able to produce second order harmonic waves with maximum amplitude. Apart from that, due to the elevated values of Polarizability and hyperpolarizability, the present compound is able to bind with other molecules with less amount of binding energy.
| TD-DFT/B3LYP/3-21G(d,p) | DMSO | Gas |
|---|---|---|
| Etotal (Hartree) | -11658.2 | -11659.6 |
| EHOMO (eV) | 6.678 | 6.570 |
| ELUMO (eV) | 3.517 | 3.419 |
| ΔEHOMO-LUMO gap[ Kubo gap (eV)] | 3.161 | 3.157 |
| Chemical hardness η (eV ) | 1.580 | 1.578 |
| Electronegativity χ(eV) | 5.047 | 4.994 |
| Chemical potential μ(eV ) | 5.047 | 4.994 |
| Electrophilicity indexω (eV ) | 7.992 | 7.899 |
| Dipole moment (Debye) | 2.213 | 2.098 |
Table 8: Calculated energies values of In2O3 solvent (DMSO) and gas phase
| Parameters | a.u | parameters | a.u |
|---|---|---|---|
| μx | 0.00 | β xxx | 0.00 |
| μy | 0.00 | β xxy | 0.00 |
| μz | 2.098 | β xyy | 0.00 |
| μ0 | 2.098 | β yyy | 0.00 |
| αxx | -58.34 | β xxz | 1.879 |
| αxy | 0.00 | β xyz | 0.00 |
| αyy | -96.63 | β yyz | 0.00 |
| αxz | 0.00 | β xzz | 0.00 |
| αyz | 0.00 | β yzz | 0.00 |
| αzz | 8.96 | β zzz | 4.64 |
| αo | 49.23 | β | 115.04 |
| Δα | 55.47 | - | - |
Table 9: The dipole moments μ (D), the polarizability α (a.u.), the average polarizability αo (esu), the anisotropy of the polarizability Δα (esu), and the first hyperpolarizability β (esu) of In2O3
Chemical properties
The Chemical properties can be used for building chemical classifications. They can also be useful to identify an unknown substance or to separate or purify it from other substances. Materials sciences normally considers the chemical properties of a substance to guide its applications. The chemical hardness and potential, electronegativity and Electrophilicity index were calculated and their values were shown in Table 8.
The chemical hardness is a good indicator of the chemical stability. The chemical stability means the thermodynamic stability of the chemical system. Normally, the metal oxides are having rich chemical stability. The chemical hardness of the present compound is same (1.58-1.57) when going from Gas to solvent. When the thin film material is made up of In and O atoms, the chemical stability character is enhanced. So the present thin film compound retains its useful properties on the timescale of its expected usefulness.
Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself [45]. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. The electronegativity is associated more with different functional groups than with individual atoms, it is called group electronegativity. The calculated electronegativity is 5.04 and if the value is greater than 1.7; the property of bonds in the molecule will be changed from covalent to ionic. Accordingly, due to the addition of In with O, the bonds character of the compound rehabilitated to ionic.
Electrophilicity index is a factor which is used to measure the energy lowering due to maximal electron flow between donor [HOMO] and acceptor [LUMO]. From the Table 8, it is found that the Electrophilicity index is 7.99 which is very high and this value ensured that the strong energy transformation is taking place between HOMO+1 and LUMO- 1 instead of HOMO-LUMO. From this value of Electrophilicity index, it is found that, constrain for electrical and optical energy flow is very less in this thin film.
The dipole moment of a molecule is another important electronic property which is the measure of electrical polarity of a system of charges. The dipole moment is the vector sum of the all bond moments in a molecule. Whenever the molecule would have possessed large dipole moment, the intermolecular interactions are very strong. The calculated dipole moment value for the title compound is 2.21 Debye in gas and 2.09 in solvent. It is moderate and inferred that; the present molecule is highly polar where the strong intermolecular interactions taking place.
Thermodynamic properties
Normally, the thermo dynamical analysis on aromatic compound was very important since they provide the necessary information regarding the chemical reactivity. On the basis of vibrational analysis at B3LYP/3-21G(d,p) level the standard statistical thermodynamic functions: standard heat capacities (C0 p,m) standard entropies (S0 m), and standard enthalpy changes (ΔH0m) for the title compounds were obtained from the theoretical harmonic frequencies and listed in Table 9. From Table 10, it can be observed that, the thermodynamic functions were increased with temperature ranging from 100 to 1000K due to the fact that the molecular vibrational intensities increased with temperature. At lower and higher temperature, the specific heat capacity of the present compound obeyed the Debye T3 law. The value entropy and enthalpy of the present compound gets elevated and saturated at 1000°K.
| T(K) | 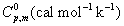 |
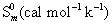 |
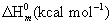 |
|---|---|---|---|
| 100.00 | 328.23 | 106.63 | 7.46 |
| 200.00 | 408.62 | 126.61 | 19.17 |
| 298.15 | 462.37 | 143.14 | 32.44 |
| 300.00 | 463.26 | 143.41 | 32.70 |
| 400.00 | 506.28 | 155.40 | 47.69 |
| 500.00 | 541.86 | 163.21 | 63.65 |
| 600.00 | 572.10 | 168.31 | 80.24 |
| 700.00 | 598.31 | 171.74 | 97.25 |
| 800.00 | 621.41 | 174.12 | 114.55 |
| 900.00 | 642.02 | 175.84 | 132.05 |
| 1000.00 | 660.62 | 177.10 | 149.70 |
Table 10: Calculated thermo dynamical parameters of In2O3 between 100-1000° K
In2O3 films were successfully deposited by spray pyrolysis technique using Indium chloride as a precursor. Initially the films were deposited for various precursor concentrations. The film deposited at 0.1M has (222) reflection as preferred orientation and improved crystallinity with maximum grains size and minimum micro strain and dislocation density. Then the films were deposited for different substrate temperatures by keeping molarity as constant as 0.1M. The results indicate that the film deposited for 0.1M at 500°C has the optimum properties with smooth surface, improved crystallinity, high band gap energy of 3.661eV, high refractive index, low extinction coefficient and high intense PL emission. This makes it the most appropriate film for gas sensing and optoelectronic applications. The complete vibrational analysis has been done from the observed peaks in FT-IR, FT-Raman spectra and UV-Visible spectra. On the basis of the calculated and experimental results; assignment of the fundamental frequencies are examined. The equilibrium geometries, harmonic wavenumbers at TD-DFT calculations are carried out. The results of computational investigation supported the physicochemical property analysis of In2O3 thin film. From the computational study, it was observed that, the remarkable change of physical and chemical properties of present compound through the electronic reconfiguration by adding of substitutional groups. The lowering of the HOMO–LUMO energy gap has substantial influence on the intra molecular charge transfer which explored the bioactivity of the molecule. The correlations between the statistical thermodynamical parameters with temperature are also obtained. It is seen that the heat capacities, entropies and enthalpies increased with temperature owing to the increasing of intensities of the molecular vibrations. The MEP maps show that the negative potential sites are on electronegative oxygen atoms and the positive potential sites were mostly around the hydrogen atoms.