Internal Medicine: Open Access
Open Access
ISSN: 2165-8048
+44 1300 500008
ISSN: 2165-8048
+44 1300 500008
Research - (2022)Volume 12, Issue 4
Background: It is of great significance for clinical diagnosis, prevention and treatment of osteoporosis to deeply understand the pathogenesis and development process of osteoporosis through animal models of osteoporosis. This systematic review aims to summarize the modeling methods of osteoporosis, reveal the current situation and progress of animal models of osteoporosis, and compare the advantages and disadvantages of various modeling methods, so as to provide reference for clinical research.
Methods: CNKI, CBM database, VIP database, Wan fang database, PubMed database and EMBASE database were searched by computer from the database establishment to December 2020 with the key words of "animal model and osteoporosis" in Chinese and English respectively. The literatures were screened according to inclusion and exclusion criteria. The methods of osteoporosis modeling, the improvement of the methods and the advantages and disadvantages of each method are summarized.
Discussion: A total of 9303 related literatures were collected, and 112 eligible literatures were included. The establishment of an appropriate animal model is the key to the etiology, pathophysiology and drug therapy of osteoporosis. As the causes and pathophysiological changes of different types of OP have their own characteristics, the modeling methods are also different. Therefore, different modeling methods and experimental animals should be selected according to different experimental requirements.
Animal model; Ovariectomy; Primary osteoporosis; Secondary osteoporosis
Osteoporosis is a slow progressing disease and characteristics are the deterioration of bone tissue, loss of bone mass, bone fragility and increasing fracture risk [1-3]. Osteoporosis usually has no obvious symptoms, with little or no trauma in some cases, unless it deteriorates into a fracture [4]. Bone fractures occur every three seconds as a result of osteoporosis, and spinal and hip fractures cause a large number of disability (4.48 million in 1990) [5], and morbidity worldwide [6].
Osteoporosis is generally classified as primary or secondary. Primary disease is due to the sudden decrease of sex hormones and physiological degenerative changes caused by age, including juvenile idiopathic osteoporosis. Postmenopausal and senile osteoporosis is the most prevalent types in humans. Secondary osteoporosis, which is about 10% of the total number of cases, can be induced by diseases or drug factors, such as endocrine and metabolic diseases (diabetes and hyperthyroidism), kidney diseases, liver diseases and gastrointestinal pathologies. Drugs can induce osteoporosis, including long-term high-dose heparin, immune suppressants, anti-epileptics and glucocorticoids. Endocrine disorders, lifestyle factors and long-term immobilization are also causes of osteoporosis [7,8].
It is essential to conduct preclinical studies using animal models that mimic the relevant features of human disease processes. Animal models can play a very important role in the study of osteoporosis. The pathogenesis and pathological mechanisms of osteoporosis have independent characteristics and animal models of osteoporosis therefore vary. Proper study of osteoporosis using animal models will enhance understanding of the mechanisms of many treatment methods and allow the development of preventive and therapeutic drugs.
Data sources and searches
The CNKI, Wan fang, VIP, Web of Science, PubMed and EMBASE databases were searched. The key words were osteoporosis and animal model in both Chinese and English. The retrieval time was from the establishment of the database to December 2020.
Inclusion and exclusion criteria
Inclusion criteria: 1) the content of the literature was closely related to the subject of this study; 2) the articles were on animal models of osteoporosis; and 3) the articles described animal experiment.
Exclusion criteria: 1) repetitive and obsolete articles unrelated to the content of this study; 2) studies employing non-simple animal modelling; and 3) conference abstracts, comments, narrative comments and case reports.
Study selection
Two reviewers independently screened the titles, abstracts and full-text for inclusion. Differences were resolved by consensus through consultation. A third reviewer was consulted if there were any unresolved discrepancies between the reviewers at any stage in the article selection process. Next, data extraction was conducted independently by the two reviewers, and the results of data extraction were compared again.
Data abstraction and quality assessment
Data extraction was done back-to-back by the two reviewers using a spreadsheet to extract the following data: First author, publication date of the article, country, selected animals, modelling methods, Bone Mineral Density (BMD) measurement locations and methods, experimental observation time, detection indicators and other factors (Figure 1).
Search results
The process of selecting the studies is described in Figure 1. We retrieved 9,303 records in our search. After reviewing the abstracts and full texts, we included 112 studies. Among them, 53 were from Chinese databases and 59 were from English databases. The most common method of modelling was ovariectomy for females and testicle removal was performed in males [9-21]. The second method was the animal model of castration combined with drugs [1,22-35]. A few other modelling methods, such as drug modelling alone [28,36-43], nutritional deficiency modelling [44-48], disuse modelling [16,49,50] and gene knockout modelling [51-56] were used in the retrieved studies (Table 1).
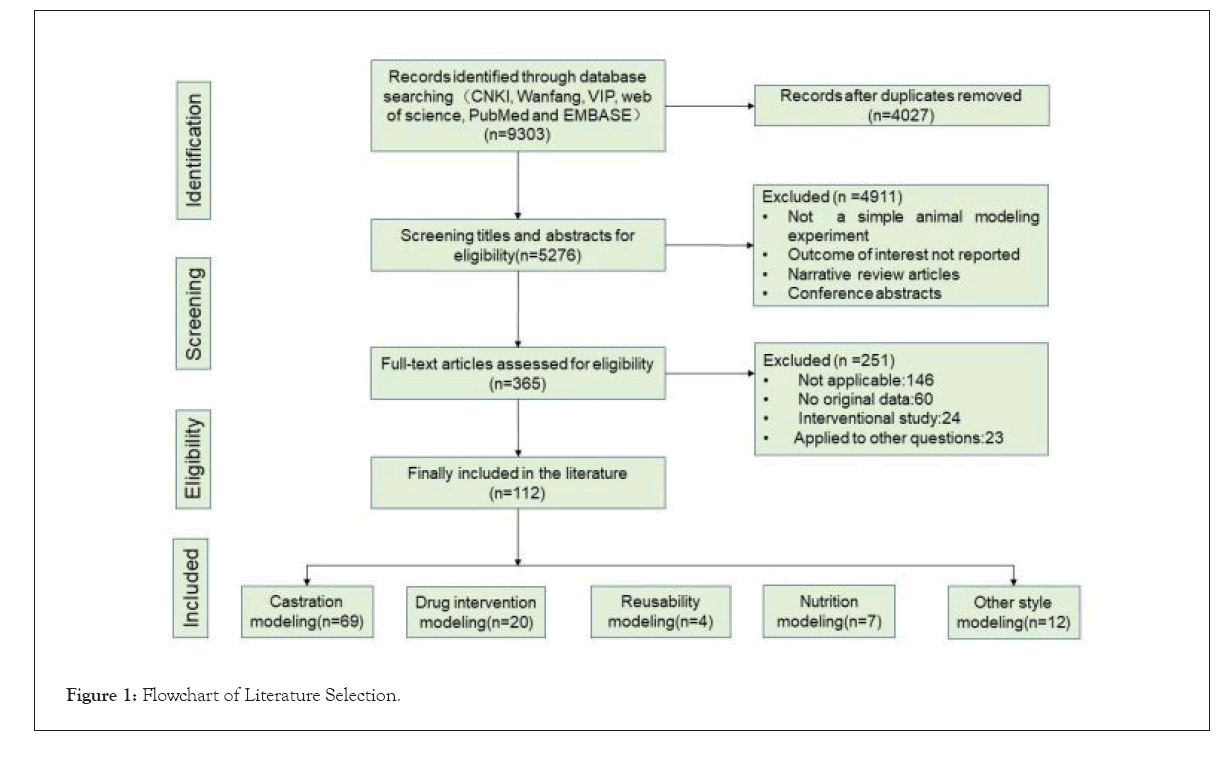
Figure 1: Flowchart of Literature Selection.
| Reference | Animal | Animal Model(Duration) | Animal No. | Outcome measures | ||
|---|---|---|---|---|---|---|
| BMD Location | BMD Measurement | Others | ||||
| Guo, 2019 [1] | Rats | OVX+ERK-5 | 60 | Femurs | DXA | Ca, P, ALP, Three-point bending test, biomechanical property |
| (8 weeks post-operatively) | ||||||
| Lin, 2020 [27] | Rabbit | GC | 56 | Femurs | DXA | Tb. Th, Tb.Sp, Tb.N, BS/BV, BV/TV, Ca, P, TC, TG |
| (2, 4, 8 weeks post-operatively) | ||||||
| Hui, 2018 [68] | Rats | exposed to silica | 12 | Femurs, tibia | micro-CT | Tb.Th, Tb.Sp, Tb.N, SMI, Ca, P, PTH, 25-(OH)-D, etc. |
| (24 weeks) | ||||||
| Liu, 2014 [43] | Rats | OVX | 33 | whole body | micro-CT | ~ |
| (2, 4, 12, 24 and 36 weeks post-surgery) | ||||||
| Huang, 2016 [53] | Rats | crp+db/db | 33 | tibia | micro-CT | SMI, Tb.Th, Tb.Sp, Tb.N |
| (36 weeks) | ||||||
| Egermann, 2011 [25] | Sheep | OVX+Px | 26 | distal radius | pQCT | Tb.Th, Tb.Sp, Tb.N, BS/BV, BV/TV, bone histological parameters, Bone markers |
| (before and 3, 9, 18 and 30 months after surgery) | ||||||
| Stendig-Lindberg, 2014 [8] | Rats | Mg | 16 | Femurs, lumbar | DXA | BV/TV, , TBPf, Mg , etc. |
| ( 12 months) | ||||||
| Oheim, 2012 [31] | Sheep | OVX+CSF/Leptin-LV/Leptin-TV | 16 | No | No | BV/TV |
| (3 months) | ||||||
| Schulz, 2017 [41] | Pigs | Prednisolone | 37 | Lumbar, mandible and maxilla | QCT | serum parameters |
| (before and after 6, 9 months) | ||||||
| Goldhahn, 2005 [26] | Sheep | OVX+ MP | 18 | Distal radius | pQCT | BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N, SMI |
| (before OVX and after 12, 17, 22, 27, and 40 weeks) | ||||||
| Oheim, 2013 [30] | Sheep | OVX+HPD | 5 | No | No | BV/TV, Tb.Th, Tb.Sp, Tb.N, Histo morphometry, Biomechanical Testing, etc. |
| (12, 24 mouth after surgery) | ||||||
| Kurth, 2001 [54] | Rats | W256 | 30 | No | No | ctBMC, tBMC, BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N |
| (28 days after surgery) | ||||||
| Eschler, 2015 [29] | Sheep | OVX+DEX | 24 | distal radius, lumbar | pQCT | BV/TV, Tb.Th, Tb.Sp, Tb.N, SMI |
| (5.5 months) | ||||||
| Chen, 2009 [51] | Mice | SAMP6 | 32 | Femurs, tibia, lumbar | micro-CT | BV/TV, Tb.Th, Tb.Sp, Tb.N, TBPf, SMI, etc. |
| (5, 12 months) | ||||||
| Shen, 1997 [24] | Rats | OVX, LoCa, IM | 84 | No | No | BV/TV, Tb.Th, Tb.Sp, Tb.N, etc. |
| (4 weeks) | ||||||
| Wu, 1990 [46] | Rabbit | LoCa | 10 | No | No | Ca, P, BMC, Tb.Th, Tb.N, etc. |
| (14 weeks) | ||||||
| Dick, 1996 [52] | Rats | OOX | 36 | Global, Femurs, lumbar | DXA | BMC |
| (1, 3, 6 weeks after surgery) | ||||||
| Sevil, 2010 [17] | Rabbit | OVX | 24 | Femurs | DXA | BMC, BA, weight, Femur(Cortical thickness, Diameter, Area), three-point bending |
| (8 and 16 weeks after surgery) | ||||||
| Peng, 1994 [56] | Rats | OVX | 97 | No | No | Weight, Ash weight, ash weight/body weight , bone volume, length of Femur, Stress and strain, diameter, conical bone area, and bone marrow area |
| (6 weeks), IMM(3 weeks) | ||||||
| Fini, 2000 [12] | Rats | OVX | 12 | No | No | BV/TV, Ce.V/TV, Tb.Th, Tb.Sp, Tb.N, O.Th, OV/TV, MAR, BFR/BS, Aj.AR, Mlt, Omt |
| ( 12 and 24 months after surgery) | ||||||
| Noor, 2014 [11] | Rats | OVX | 30 | No | No | BV/TV, Tb.Th, Tb.Sp, Tb.N, CTX, bone mineral elements(Ca, P, Fe, Cu, Zn, Ni, Ca/P, Cu/Zn), |
| (4, 8 weeks after surgery) | ||||||
| Matsushita, 1986 [45] | Mice | SAM | 229 | No | No | Femur(Ca, P), HYP |
| ( 4 or 5 months of age) | ||||||
| Wanderman, 2018 [16] | Rabbit | OVX | 36 | tibia, Femur | DXA | weight |
| (17 weeks postoperatively) | ||||||
| Kreipke, 2014 [15] | Sheep | OVX | 13 | vertebral, Femur | μ-CT | BV/TV, TMD, SMI, Tb.Sp, Tb.Th, DA |
| (12, 24 months after surgery) | ||||||
| Muller, 2019 [35] | Sheep | OVX, O+Lo.Ca, O+Lo.Ca+GLU | 28 | lumbar | DXA | BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N, SMI, etc. |
| (8 months ) | ||||||
| Sipos, 2011 [95] | Cows | OVX+Lo.Ca | 32 | Femur | DXA | BV/TV, BS/BV, Tb.N, Tb.Th, Tb.Sp, DA, Conndens, SMI, cytokine genes, Biochemical bone marker , etc. |
| (10 months) | ||||||
| Nakano, 1996 [38] | Rats | CCI4+TAA | 126 | No | No | Histo morphometric parameters, Biological parameters, Biochemical parameters, Bone mineral metabolism parameters, etc. |
| (8, 12 and 24 weeks after start) | ||||||
| Isomura, 2003 [40] | Rats | iron lactate | 48 | No | No | weights, ALP, Ca, P, Fe, Osteocalcin, Osteopontin, Deoxy pyridinoline, etc. |
| (4 weeks after diets) | ||||||
| Ryu, 2015 [14] | Rats | OCX | 20 | Femur | Micro CT | ~ |
| (8, 10 weeks post-surgery) | ||||||
| Castañeda, 2006 [28] | Rabbits | OVX+corticosteroid | 29 | lumbar, knee, tibia | DXA | BA, BMC |
| (4 weeks after surgery) | ||||||
| Xiao, 2015 [36] | Rats | STZ | 140 | No | No | Ca, P, AKP, BGP |
| ( 4, 8, 12, and 16 weeks after first injection of STZ) | ||||||
| Li, 2018 [68] | Rabbits | OVX+MP | 32 | lumbar | Micro CT | weight, BMD, BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N, etc. |
| (6, 10 weeks after surgery) | ||||||
| Harrison, 2020 [27] | Rabbits | OVX, GC, OVX+GC, PTH(12 weeks) | 35 | No | No | BV/TV, Tb.Th, Tb.Sp, Tb.N, etc. |
| Stolzing, 2010 [39] | Rats | STZ | 8 | tibia | Micro CT | BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N, etc. |
| (1, 4, 12 weeks) | ||||||
| Amanzadeh, 2003 [44] | Rats | casein-diet | 36 | No | No | Bone histo morphometry, Serum biochemistry, Urinary stone risk factors, etc. |
| (60 days) | ||||||
| Maryin, 1976 [10] | Beagle dogs | Lo.Ca+Lo.P | 15 | No | No | Ca, P, Mg, etc. |
| (6-16 months) | ||||||
| Keiler, 2012 [13] | Rats | OVX | 8 | tibia, lumbar | Micro CT | BV/TV, Tb.Th, Tb.Sp, Tb.N, Conn.D |
| (10 weeks after surgery) | ||||||
| Melville, 2014 [55] | Mice | Transgene | 33 | tibia, Femur, lumbar | Micro CT | BV/TV, Tb.Th, Tb.Sp, etc. |
| (4, 8, 12, and 18 weeks of age) | ||||||
| Schorlemmer, 2003 [23] | Sheep | OVX, OVX+GLU | 16 | tibia, lumbar | pQCT | BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N, ON/BV, OS/BS |
| (12 months) | ||||||
| Lin, 2014 [43] | Rats | GLU | 36 | lumbar, Femur | DXA | BMC, MA, SMI, BV/TV, Tb.Th, Tb.N, Tb.Sp, etc. |
| (3 months) | ||||||
| Cabrera, 2018 [34] | Sheep | OVX+GLU | 28 | lumbar, Femur, tibia | DXA+pQCT | BMC, OC, CTx-1 |
| (5 month after surgery) | ||||||
| Yang, 2003 [21] | Rats | OVX | 28 | tibia | Micro CT | BV/TV, Tb.Th, Tb.Sp, SMI |
| (6, 16 weeks after surgery) | ||||||
| Fleming, 2005 [42] | Zebrafish | skeletal staining | 15 | No | No | vitamin D3 analogs, PTH |
| (6 days) | ||||||
| Hanyu, 1999 [37] | Rats | bovine type II collagen | 35 | No | No | BV/TV, Tb.Th, Tb.N, OV/TV, etc. |
| (4, 6, 14 weeks) | ||||||
| Aguado, 2017 [49] | Chicken | Limit movement | 20 | No | No | BV/TV, Tb.Th, Tb.Sp, Tb.N, SMI, Tb.Pf |
| (53 days of age) | ||||||
| Wang, 2015 | Mice | OVX, OVX+Fe | 24 | No | No | AKP, TRAP, ROS, BV/TV, Tb.Th, Tb.Sp, Tb.Pf, SMI, RUNX2, SP7, BGLAP, etc. |
| (2 months for feed) | ||||||
| Jiang, 2013 [50] | Rats | SCI, hind limb cast-immobilized | 18 | No | No | weight, ALPase activity, PICP and osteocalcin, Run, osterix |
| (3 weeks) | ||||||
| Leitner, 2009 [20] | Rats | OVX | 21 | Femur | pQCT+MicroCT | BV/TV, VOI |
| (4 weeks for surgery, 4 weeks after surgery been euthanized) | ||||||
| Miller, 1993 [9] | Rats | OVX | 24 | No | No | Type and length of trabecular struts, Marrow star volumes |
| (18 months after surgery) | ||||||
| Rude, 2003 [48] | Mice | diet-lo.Mg | 23 | No/Femur, tibia | No | Serum Mg, Ca, PTH, and/or TNFa, Ash(Mg, Ca, P), Histo morphometric indices(BV/TV, etc.) |
| (1-6 weeks) | ||||||
| Oheim, 2014 [94] | Sheep | OVX+HPD | 10 | No | No | Histo morphometry (BV/TV, Tb.Th, Tb.N, Tb.Sp, OV/BV, OS/BS, etc.) |
| (6 months) | ||||||
| Ferretti, 2015 [47] | Rats | lo.Ca+PTH | 18 | No | No | BV/TV, Tb.Th, Tb.Sp, Tb.N, Ct.Th, Ct-B-Ar, Ca, P, OPG, BALP, PTH, etc. |
Table 1: Table of general characteristics of the included english studies.
Selection of experimental animals for research model
It is very important to select the correct osteoporosis model animal. Different osteoporosis models, including animal species and human bone tissues, have different histological and biological metabolism characteristics. Measurements of basic bone mass vary for different ages and genders, and choosing the animal species for an osteoporosis model is very strict. At present, the commonly used animals include rats, mice, dogs, sheep, rabbits, pigs and primates [57-61]. Rodents (including rats and mice) have been used in over 60% of the studies, followed by sheep and rabbits, according to the results of our survey. Other non-human primates, such as pigs and dogs, have also been used, but in relatively small numbers.
It is very important to select the correct osteoporosis model animal. Different osteoporosis models, including animal species and human bone tissues, have different histological and biological metabolism characteristics. Measurements of basic bone mass vary for different ages and genders, and choosing the animal species for an osteoporosis model is very strict. At present, the commonly used animals include rats, mice, dogs, sheep, rabbits, pigs and primates [57-61]. Rodents (including rats and mice) have been used in over 60% of the studies, followed by sheep and rabbits, according to the results of our survey. Other non-human primates, such as pigs and dogs, have also been used, but in relatively small numbers.
Because of the clear understanding of the mouse genome, mice have become a common experimental animal in the field of bone mass gene control. Mice are often used to study the genetic factors of bone metabolism, importing or knocking out target genes, and observing phenotype and pathological changes. Mice are primary experimental model animals in the study of influential genetic factors on peak bone mass and age-related bone loss [62]. However, the disadvantages are: The epiphysis closure is slow and the bone reconstruction cycle is shorter than that of humans, which may interfere with the experimental results. Because ovariectomy does not cause brittle fractures, it is not suitable for the study of cortical bone changes after ovariectomy. The life cycle of rats is short and the blood volume is small so it is often impossible to take blood and biopsy samples. The biological cycle of rodents is significantly different from that of humans and this may produce errors in experiments. These issues draw attention to the need for models that more closely mimic humans [64]. The lack of a Haversian reconstruction system and low activity of cortical reconstruction is not suitable for evaluating drugs promoting the role of Haversian reconstruction [63,65].
Compared with rodents, adult rabbits have obvious haversian system reconstruction ability, a faster bone turnover rate [16] and earlier epiphyseal closure (usually 6-8 months) [7,66]. According to the results of the included literature, rabbits have been used more often for ovariectomised, glucocorticoid and ovariectomised+glucocorticoid osteoporosis animal modelling [16,17,27,28,32]. Other animals, such as sheep, pigs, dogs [62] and non-human primates also have a Haversian reconstruction system, and non-human primates are genetically closer to humans and have similar oestrus cycles [64]. However, they are expensive [22-67], difficult to manage [31,57], and hormonal changes have little impact on bone loss [10,61], so they are not used in models of osteoporosis.
Ideal experimental animal models would include absolute replication of human diseases. Unfortunately, this goal has not been achieved in the study of osteoporosis. Rats, mice, dogs, monkeys and apes are the main animals that have been used to simulate osteoporosis. Each species has its own advantages and disadvantages and not one of the experimental animal species includes all risk factors associated with an osteoporosis model [62].
The basis for judging the effect of the modeling method
Since the lumbar spine, femur and tibia are the most common clinical fracture sites, most experiments have measured the BMD and Bone Mineral Content (BMC) in these bones. The main measurement method is scanning with a Dual-Energy X-ray Absorptiometry (DXA) instrument. Next, bone histo metrics, biochemical parameters of blood samples (eg. calcium and phosphorus), and bone biomechanics indexes have been determined. For postmenopausal osteoporosis, because the incidence of this type of osteoporosis is related to a decrease in oestrogen, the estradiol can be increased when comparing various indicators. All the above indicators can reflect the osteoporosis situation in animals from different aspects. It is necessary to comprehensively analyze a variety of indicators to achieve a comprehensive judgement on modelling effects.
Primary osteoporosis model
Post-Menopausal Osteoporosis Model (PMOP): The OVX rat model is a classic model of PMOP [68] and it has been widely adopted to mimic oestrogen-deficiency-induced bone loss [18]. OVX can induce a decrease in oestrogen levels and increase the recruitment, differentiation and survival of osteoclasts so that bone resorption exceeds bone formation, which eventually leads to osteoporosis [7].
In this model, bilateral ovaries are resected in a sterile environment, which results in a rapid decrease of oestrogen, enhanced bone turnover and increased bone resorption, resulting in osteoporosis [69]. The bone mass loss after modelling was mainly trabecular bone loss, which is similar to the bond loss in postmenopausal women. The Food and Drug Administration (FDA) has suggested that rats aged 6–10 months should be used to establish the model, which is usually 12 weeks or longer after castration [70]. Surgical castration has been widely used because of its single modelling factor, definite modelling effect, good repeatability, high reliability of experimental results, and it can accurately reflect the cause of oestrogen decline, which successfully simulates the characteristics of postmenopausal osteoporosis bone metabolism.
However, there are still some controversies. First, oestrogen levels in animals suddenly and rapidly decrease after oophorectomy, while oestrogen decreases are long and slow processes in the natural course of disease, and ovarian stromal cells in postmenopausal women still have some endocrine function. Second, surgical castration itself is traumatic, which may cause a negative nitrogen balance, stress response and electrolyte disorder, which may affect the detection of indicators. Third, ovarian resection may lead to weight gain in rats, and weight gain may partially protect against bone loss. Finally, when oestrogen replacement therapy was studied in a castrated rat model, the fertility status of the rats affected the experimental results.
In addition, the removal of male testis can also be used to construct an osteoporosis model. These models have been used in the study of basic theory and drug intervention in male osteoporosis and significant results have been achieved. However, due to the continuous growth of the adult male epiphysis after 30 months, the experimental results using male rats are not widely accepted by scholars.
The effect of OVX on bone is not consistent in different bone sites [71]. Loss in long bones, including the tibia, femur, humerus and ulna, was reported at 36 weeks after ovariectomy (75.0%, 70.4%, 64.9% and 57.1%, respectively), compared with that of the lumbar spine and iliac bone (36.6% and 51.6%, respectively) [18]. In addition, only the ulna, femur and tibia showed significant bone loss at four weeks after OVX, indicating that these areas were more sensitive to OVX [18]. Also, OVX-induced bone loss is more severe and observed earlier in the proximal tibia than in the lumbar spine or femur, so short-term studies of the proximal tibia are recommended [71]. The use of rabbits [16,17], rodents (rats and mice) [11-13,18,21], sheep [15] and non-human primates as animal models of OVX osteoporosis is recommended. We prefer rabbits and rats for reasons of economics, experiment time and animal ethics.
Senile osteoporosis model: Primary osteoporosis includes postmenopausal osteoporosis and senile osteoporosis. In both males and females, the loss of cancellous bone (also known as trabeculae) begins at thirty years old and there is rapid loss during menopause. On the other hand, most cortical bone loss occurs at 10 years after menopause due to cortical thinning and increased cortical porosity [60,71]. Bones in most mammals are thought to deteriorate with age, but in animals commonly used in biological research, age-related bone loss is only well documented in crab-eating monkeys [64], sheep (6~10 years old) [72], rats and mice [73-77].
Rats typically live two to three years, with bone mass peaking at 4–8 months of age and then declining with age [78]. Watanabe et al. [78] introduced several classic models of age-related osteoporosis in which the strains of mice were C57BL/6, BALB/C and Senescence Accelerated Mice (SAM) [74-76]. However, inbreed mice (C57BL/6 and BALB/C) were prone to die of cancer [79], which affected the process of subsequent experiments. Takeda [80] and his colleagues established a SAM composed of SAMP and SAMR series. Compared with normal mice, SAMR and SAMP had accelerated ageing. Senescence-accelerated mouse prone 6 (SAMP6) was reported as the first mouse model of spontaneous senile osteoporosis [76]. Only one of the articles included in our study used SAMP6 rats as model animals [51]. Azuma et al. [74] found that SAMP6 mice have many morphologic and molecular features that mimic human bone ageing, and they are considered as a useful experimental model for the spontaneity of age-related osteoporosis [76].
Gene recombination animal model of osteoporosis: Developments in genetic technology have made it possible to create animal models with specific genetic traits by silencing or knocking out a particular gene. Gene technology used in osteoporosis modelling mainly includes gene knockout technology and gene mutation technology. Most of the mice treated with gene technology were used to study primary osteoporosis. For example, osteoprotegerin knockout mice (OPG-/-) [81], were used to study postmenopausal osteoporosis, and gene mutation mice SAMP6 were used to study ageing osteoporosis [75]. Some studies reported that various genes, cytokines and pathways were associated with BMD, osteoporosis, or fractures [82-84]. Compared with the classical castrated rat model, SAMP6 has obvious advantages. It has a clear genetic background and avoids interference from the external environment as much as possible. Osteoporosis can occur in the early postnatal period, which can shorten the experiment time. Without surgical intervention, the negative nitrogen balance of the model animals did not occur, and there was little influence on the internal environment related to sex hormones in vivo. However, its high price, complicated process and technical difficulty undoubtedly limit its application.
Secondary osteoporosis model
Glucocorticoid Osteoporosis (GIOP): Due to the wide application of glucocorticoids in the clinic, the incidence of osteoporosis caused by glucocorticoids is second only to postmenopausal osteoporosis and senile osteoporosis, and ranks first in secondary osteoporosis. Induction methods include gavage, oral administration and intramuscular injection. The treatment drugs usually mediate prednisone; e.g. methyl prednisone and occasionally high-potency dexamethasone. Significant bone loss occurs as early as 10 days and as late as 48 weeks after use. However, this model is not fully suitable for evaluating the inhibitory effects of drugs on bone resorption, because glucocorticoid-induced osteoporosis is not consistent with the pathogenesis and course of primary osteoporosis. Rats, mice, rabbits [85], sheep [26], pigs [41] and dogs can be used as models in this method. The rabbits are sensitive to glucocorticoid induction, and the modelling time is short [32]. Rats are usually the dominant model and can be successfully modelled after 5–6 weeks.
Retinoic Acid (RA): Retinoic acid, a derivative of vitamin A, can activate osteoclasts and promote bone resorption, but it does not inhibit the activity of osteoblasts and has no obvious effect on bone formation and the mineralization process of the bone matrix. As a result, bone remodeling is in a negative balance state with bone resorption greater than bone formation, and this ultimately leads to osteoporosis in animals. Although the pathogenic factors of this model are different from the clinical factors, it is similar to humans regarding symptoms, histo morphological manifestations and bone responses to oestrogen. In addition, it is a commonly used modelling method for acute osteoporosis in rats due to its short modelling time [86,87]. Generally, oral administration of retinoic acid or a gavage of 70–105 mg/kg for two consecutive weeks can successfully establish an osteoporosis model, which has good short-term effects but poor long-term effects. This model has the advantages of convenient operation, a high success rate and typical symptoms [86], so it is widely used in the research and development of new drugs.
Alcoholic osteoporosis model: The abuse of alcohol is one of the most important lifestyle risk factors for osteoporosis. Microanatomical changes in the skeletons of alcohol-dependent rats were later identified in human alcoholics, providing evidence that rats are useful for forecasting human outcomes. The use of this model has brought a better understanding of the pathogeny and severity of alcohol-induced bone loss. Excessive intake of ethanol can cause bone loss [62], increased adipose tissue in the bone marrow, altered numbers and activities of osteoblasts and osteoclasts, and increased apoptosis of bone cells, which can lead to secondary osteoporosis [88,89]. At present, the ethyl alcohol model is only used to study the mechanisms of alcohol-induced osteoporosis.
Disuse osteoporosis model: Disused osteoporosis models, which include surgical and non-surgical methods, are of great significance in the study of osteoporosis in paralysis, long-term postoperative bedridden cases, and aviation personnel [90,91]. Surgical methods include denervation [92], tendon removal and spinal cord resection. Non-operative methods include suspension [93], bandage binding and screw fixation. Rats have been extensively used as a model for disuse osteoporosis [91-93]. Each of these seemingly disparate methods led to similar skeletal changes, implying that the principal impacts on bone loss are due to pressure unloading. These models have been used to study the pathogeny of disuse osteoporosis rats in growth stages and maturity, as well as to evaluate the efficacy of potential interventions [62]. The results of Peng et al. [56] showed that, compared with a control group, the maximum load on the femoral neck was significantly reduced (27.7%, P<0.001) and the energy absorption was significantly decreased (45.3%, P<0.001) with Immobilisation (IMM).
Brain-derived osteoporosis model: The hypothalamo-pituitary gland system regulates the balance of several hormones, such as thyroid hormones (T3, T4), gonadotropins (LH, FSH), cortisol and leptin and Insulin-like Growth Factor-1 (IGF-1). Hypothalamic-Pituitary Dissection (HPD) results in significant bone loss in sheep, affecting the trabeculae and cortical bone [94]. Oheim et al. [30] showed significant trabeculae and cortical bone loss at 24 months after HPD in sheep. Histo morphometric analysis of the iliac crest showed a significant 60% reduction in BV/TV compared to the control group. Melatonin secreted by the pineal gland can affect bone metabolism. A study by Egermann et al. [25] found that bone absorption increased after Pineal Resection (PX), and cancellous Bone Volume (BV/TV) decreased by −13.3% six months later and decreased by −21.5% if combined with OVX. Thirty months after surgery, there was still continuous bone loss. Although the degree of bone loss caused by this method is not up to the standard of OP (−2.5 SD), it is can be used together with other methods in the construction of osteoporosis models. In addition, lateral ventricular injection of leptin significantly reduced left ventricular bone formation and resulted in significant trabecular loss in sheep [31].
Combined osteoporosis model
Due to the diversity in osteoporosis, a combined modelling method has great practical significance and application value. The combined modelling method not only simulates a variety of mechanisms of osteoporosis, but also has the advantages of a short modelling period and good modelling effect. It has therefore been favoured by many scholars. An osteoporosis model can shorten the modelling time by using other modelling methods combined with castration, such as castration+diet [95] and castration+glucocorticoids [23,26-29,34,35,85]. Generally, the effect of combined modelling on bone mass, bone structure and biomechanical properties is more obvious than that of a single modelling method [23].
In summary, there are many factors that cause osteoporosis. Different modelling methods can be chosen according to different causes of osteoporosis, and different animal models can be chosen according to different research directions, local animal ethical requirements, project funding and other factors. Most studies use rats for the construction of an osteoporosis model. We recommend 6–9 month old rats as an animal model for postmenopausal osteoporosis [18] because the epiphysis of rats less than 6 months old has not been closed and the reduction of bone mass is not obvious [19]. Rats older than nine months will have the effect of ageing osteoporosis [13]. For some special studies, other animals can be chosen; such as knockout mice, and for fracture implants, other larger animals can be used. If the conditions permit, larger animals, such as rabbits, sheep, non-human primates and pigs, can be used in an animal model.
The exploration of osteoporosis has gradually matured. However, various experimental animals at present still have limitations and various modelling methods have their own emphasis. Animal models that have been produced can only show the characteristics of the disease in some aspects, such as aetiology, clinical symptoms, or pathophysiological changes. Each animal model has its own characteristics of osteoporosis. Researchers should, according to their own research scope, define the content they want to study in the experimental design before initiating the experiment and select the appropriate modelling method to make it resemble experiences within clinical practices. With further development, more models will be reported and this will lay the foundation for a more comprehensive and thorough study of human osteoporosis.
Ethics approval and consent to participate
Not Appicable
Not applicable
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.
No patient involved
No additional data from this study are available.
The research is funded by Sichuan Provincial Administration of traditional Chinese Medicine, the project number is 2021MS454, and the special fund for 100 Talents Program of the First Affiliated Hospital of Chengdu University of TCM, the project number is 20-Y14.
Panyun Mu and Peihua Qu performed the search for the systematic review and extracted and prepared the data for the analysis. Yunlin Li and Jie Feng provide technical guidance for this system evaluation. Panyun Mu and Yimei Hu design and drafted the work. Yimei Hu substantively revised the work. The authors have revised the manuscript, read, and approved the final version.
Not applicable
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [All versions] [PubMed]
[Cross Ref] [Google Scholar] [All versions] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Google Scholar] [All versions] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
Citation: Mu P, Qu P, Feng J, Xiong F, Hu Y, Li Y (2022) Preparation for Animal Models of Osteoporosis: A Systematic Review. Intern Med.12:371.
Received: 14-Sep-2022, Manuscript No. IME-22-19200; Editor assigned: 16-Sep-2022, Pre QC No. IME-22-19200; Reviewed: 04-Oct-2022, QC No. IME-22-19200; Revised: 11-Oct-2022, Manuscript No. IME-22-19200; Published: 19-Oct-2022 , DOI: 10.35248/2165-8048.22.12.371
Copyright: © 2022 Mu P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.