Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2022)Volume 13, Issue 5
In this study, a new amidoxime adsorbent based on waste Polypropylene (PP) fabric was prepared for the adsorption of aqueous Cr (VI) metal ions. The pre-irradiation technique was used for grafting Acrylonitrile (AN) onto PP fabric. The grafting reaction was carried out at 80°C in a water bath for 4 hours, utilizing H2SO4 as an additive using 60% AN concentration and a 30 kGy radiation dose. The amidoxime adsorbent was produced by treating Acrylonitrile grafted Polypropylene (AN-g-PP) with NH2OH.HCl and characterized by FTIR, SEM, TGA, and DMA. The adsorption of Cr (VI) was investigated under various contact time, pH, temperature, and metal ion concentration circumstances. Both the Langmuir and Freundlich isotherm models were used to fit the equilibrium adsorption data. The kinetic data follows the pseudo-first-order model, and the estimated and experimental sorption capacities are in good agreement. The thermodynamic data showed that the sorption process was endothermic and spontaneous, taking place by increasing the randomness of the system. Furthermore, the study of the desorption of Cr (VI) from the adsorbent surface and reuse of the adsorbent indicates that the adsorbent can be applied effectively for the efficient removal of Cr (VI) ions from aqueous solutions.
Adsorption; Adsorption Capacity; Desorption; Grafting; Pre-irradiation
All over the world, environmental pollution due to the discharge of hazardous metals into the water streams has become one of the most extreme problems because these pollutants are deleterious to the ecosystem and human beings [1]. Chromium is one of the poisonous elements existing in two different oxidation states in the aquatic system; trivalent chromium (Cr (III)), and hexavalent chromium (Cr (VI)) which are considerably different in toxicity, the first form is the important trace element for the metabolism in mammals and less toxic while the second form is carcinogenic and significantly toxic due to its higher solubility and mobility in water [2,3]. The poisonous Cr (VI) is discharged into the environment from leather tanning, paint, and pigments, electroplating, steel fabrication, cement, dyeing, fertilizer, photography, textile, and canning industry [4]. It is not only carcinogenic but also genotoxic and hemotoxic [5]. The cause of the toxicological behavior of Cr (VI) is its oxidizing ability and formation of free radicals when it is reduced to Cr (III) taking place inside the cell [6]. It is therefore very essential to eliminate traces of Cr (VI) present in the various industrial effluents before releasing them into the aquatic environment.
For this purpose, a number of physicochemical methods have been utilized. They are solvent extraction, ion-exchange, chemical precipitation, sulfide precipitation, cementation, electro-dialysis, evaporation, electrochemical reduction, reverse osmosis, and adsorption [7]. However, these methods have some demerits and the most recommended technique for the removal of precarious metals is the adsorption method due to its high efficiency, inexpensiveness, availability, and simplicity of operation [8,9]. Many researchers have been used different types of adsorbents including bimetallic-carbon nano composites [10], activated carbon [11], and nano materials [12,13].
Recently, the use of graft polymer as an adsorbent for the removal of heavy metals has been drawn remarkable attention from scientists in this field [14-17]. Grating is a polymerization reaction in which various functional monomers are attached covalently to the main polymer chain. The advantage of grafting is the injection of different functional monomers possessed by the graft polymer into the parent polymer remaining unchanged the mechanical property of the main polymer [18,19]. The different graft polymerization methods include plasma treatment, UV light, ionizing radiation, decomposition of chemical initiators, and radiation-induced grafting. Among the aforementioned methods, radiation-induced grafting is advantageous to all other methods because of its simplicity of operation, no need for local heating, free radical process, mosaic grafting, etc [20].
Non-oven polypropylene is commonly used as packaging stuff and utilized in various industries for multiple purposes [21]. Now a day, polypropylene fabric is one of the visible plastic wastes in our daily life. PP fabric bags are discarded here and there informally and thus, our environment is polluted day by day. So, it is very urgent to find out a recycling process for the bags. For a feasible recycling process, it is very indispensable to set up an effective implementation of recovered PP fabrics. It can be used in the elimination of heavy metals from industrial effluents. Grafted polypropylene fibers have been used by different researchers for the removal of precarious metals from an aqueous solution [22-25].
The objectives of this present study are (1) utilization of a waste plastic material in the removal of a toxic dye (2) preparation of acrylonitrile (AN) grafted PP fabrics (AN-g-PP) using waste polypropylene fabric, (3) Amidoximation of AN-g-PP using NH2OH. HCl, (4) Application of the adsorbent for the removal of Cr (VI) from aqueous solution, (5) fitting the adsorption data to several kinetic and isotherm models, (6) studying the effect of temperature and pH on the adsorption process, and (7) investigation desorption of the metal ions from the adsorbent surface and its reuse.
Waste Polypropylene fabrics bags were collected from the local market (Bolivodro Bazar, Ashulia, Savar, Dhaka) and cut into small sizes before washing with acetone and drying in an oven at 60°C until a constant weight was obtained. The salt used for the adsorption study was K2Cr2O7 (Techno, pharm chem, India). Monomer, Acrylonitrile (AN), and hydroxylamine hydrochloride were purchased from Sigma Aldrich, USA. Acetone, 1, 5-diphenylcarbazide, sulphuric acid, and sodium carbonate were supplied by Merck, Germany.
Characterization of the adsorbent
FTIR spectrometer (AA-6800, Shimadzu, Japan) in the wavelength range 700-4000 cm-1 having a resolution 4 cm-1 was utilized to analyze functional groups attached to the adsorbent after grafting. The mechanical Property of the adsorbent was checked by DMA (dynamic mechanical analyzer), Triton technology TTDMA, UK heating the sample at the rate of 4°C in the temperature range 25°C to 200°C and retaining an oscillating frequency of 1 Hz. A Scanning Electron Microscope (SEM) of model JEOL 6400 was performed at an acceleration voltage of 10 kV to explore the surface morphology of the adsorbent. UV-Visible spectrophotometer (AA-6800, Shimadzu, Japan) was applied to estimate the concentration of Cr (VI) in the solution. To study the thermal stability of the adsorbent, TGA was used at the heating rate of 10°C/min ranging in temperature from 25°C to 600°C The source of radiation was Co-60 gamma-ray which is a Co-60 Batch Type Panoramic Irradiator from BRIT, India whose activity was 68.63 kCi with a dose rate of 13.7 kGy h-1.
Grafting of AN onto the PP fabrics
Gamma radiation from the Co-60 source was utilized to irradiate the PP fabric at a 30 kGy radiation dose at room temperature. After irradiation, the fabrics were kept in a freeze at dry-ice temperature until use. Sixty percent of AN and sulphuric acid (2% of AN) in methanol were stirred well to obtain the monomer solution. Argon gas was passed through the solution to remove dissolved oxygen. Then, the de-oxygenated monomer solution was transferred into a glass bottle carrying the irradiated PP fabrics. To avert the incorporation of air oxygen the bottle was locked firmly using a cork and kept in a water bath heated at 80 °C for 6 hours for grafting of AN onto the PP fabrics. After the reaction, the grafted fabrics were cleaned with methanol firstly and then using distilled water once and once again to wipe off the unreacted monomer and homo-polymer of AN. The amount of grafting was estimated using equation (1).

Where Wo and Wg are the dry weight of the PP fabrics and grafted PP fabrics respectively.
Amidoximation of nitrile group of AN-g-PP fabrics
An aqueous solution was prepared by dissolving 80 g of hydroxylamine hydrochloride in 1 liter distilled and sodium carbonate was added to neutralize the solution. The AN-g-PP was plunked into the solution which was then placed into a water bath warmed up at 80°C. After 4 hours, the PP adsorbent was washed with methanol and then with distilled water until the residual salts were separated. Finally, the amidoximated fabrics were desiccated in the air. The transformation of the nitrile group of AN-g-PP fabric to the amidoxime group was determined by the equation (2):

Where Wam and Wg are the weight of the amioximated and AN grafted fabrics respectively. MHA denotes the molecular mass of the hydroxylamine hydrochloride and MAN represents the molecular mass of the AN.
Adsorption of hexavalent chromium by the amidoximated adsorbent
The adsorbent obtained from the amidoximation of AN-g-PP was immersed in an aqueous solution of K2Cr2O7 of a certain volume and concentration at ambient conditions. The removal of Cr (VI) was investigated by applying various states of contact time, temperature, pH, and initial concentration of the metal ion. A particular concentration of HCl and NaOH was used to customize the pH of the solutions. A UV Spectro-Photometer was used to estimate the concentration of Cr (VI) in the aqueous solutions before and after sorption by the amidoximated AN-g-PP adsorbent; for this, a complexing agent, 1, 5 diphenyl carbazide was mixed with aqueous Cr (VI) solution in an acidic condition which develops a red-violet color solution and measured at 540 nm wavelength [26]. All the experiments were conducted in triplicate and the average value was taken. The sorption capacity of the adsorbent was calculated by the equation (3):

Where Q is the adsorption capacity in mg/g of the adsorbent, V is the volume of the solution in a liter, Co and C1 are the concentration of the metal ion in aqueous solution before and after immersing of the adsorbent respectively and W is the mass of the amidoximated adsorbent in gram.
Desorption of the hexavalent ion
Desorption studies of Cr (VI) ions from the surface of amidoximated AN-g-PP were carried out by soaking the adsorbent in a 2M solution of NaOH for 24 hours and the concentration of the metal ion concentration in the desorbed solution was measured using the UV-Visible method. The percentage of desorption was estimated using equation (4).
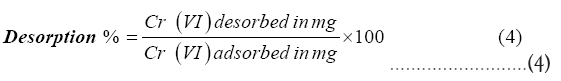
Grafting of AN onto the PP fabrics
The pre-irradiation method was applied for the grafting of AN onto the PP fabric. The advantage of this technique is that it is not required to irradiate the monomer solution i.e. only polymer backbone are irradiated so that free radicals are formed on the backbone and production of the homopolymer of monomer becomes relatively low. A Grafting reaction was taken place between irradiated (30 kGy radiation dose) polymer substrate and the monomer solution (60% AN) in methanol. Concentrated H2SO4 (2% of AN) was added to the reaction mixture as an additive. The amount of grafting in percentage was 150%.
Conversion of nitrile group into amidoxime
The pH of the NH2OH.HCl aqueous solution (80 g/L) was adjusted to 7.0 by the addition of Na2CO3 salt [27]. After the treatment of AN-g-PP with this solution for 4 hours at 80°C in a water bath, the percentage of conversion of nitrile group into amidoxime became 75%. The probable reaction mechanism for the grafting of AN onto PP and amidoximation of the AN grafted fabric could be given in Figure 1.
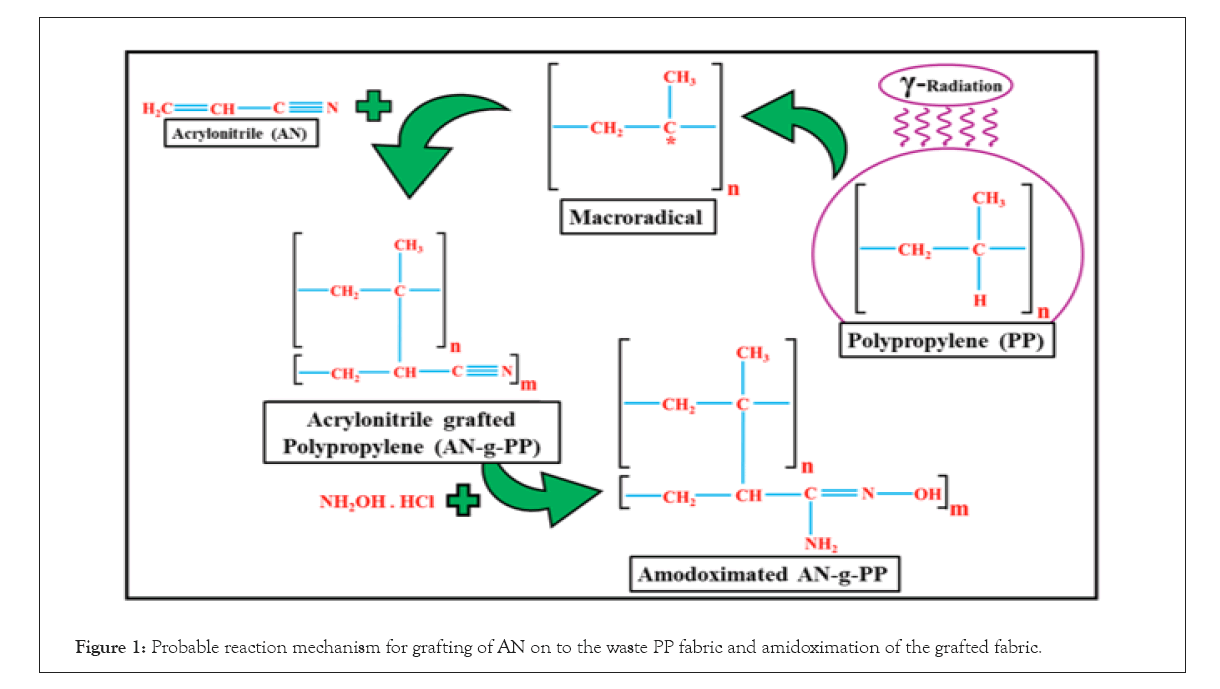
Figure 1: Probable reaction mechanism for grafting of AN on to the waste PP fabric and amidoximation of the grafted fabric.
FTIR and scanning electron microscopy analysis
Figure 2A represents the FTIR spectra of the non-woven PP fabric, AN-g-PP, and amidoximated AN-g-PP. The backbone and the side chain of the PP fabric consist of many methylenes (–CH2–) and methyl (–CH3) groups respectively. In FTIR spectra of PP fabric, the absorption bands at 2914 and 2953 cm–1 are assigned to the symmetric stretching vibrations of the methylene (CH2) and methyl (CH3) groups respectively [22]. The peaks at 2872 and 2835 cm–1 correspond to their (CH3, CH2) asymmetric stretching and the bending vibrations of these groups appeared at 1374 and 1452 cm–1 [22]. These absorption peaks of the aforementioned groups are also noticeable in the spectra of acrylonitrile grafted PP and amidoximated AN-g-PP. A sharp peak at 2243 cm–1 corresponding to the nitrile group confirms the grafting of AN onto the PP backbone. The IR spectra of amidoximated AN-g-PP obtained from the conversion of AN into amidoxime group exhibits some peaks at 1647, 1267, and 912 cm–1 which are attributed to the -C=N-, N-H, and N-O groups respectively [28]. It is observed that the peak at 2243 cm–1 corresponding to the nitrile group is reduced remarkably and the peak ascribing to the -C=N- (1647 cm–1) increases is a clear indication of the transformation of the nitrile group into the amidoxime group [29].
Scanning electron microscopy was done to inspect the surface morphology of PP fabric, AN-g-PP, and amidoximated AN-g-PP as shown in Figure 2B, 2C, and 2D. After pre-irradiation grafting of AN, a substantial change in the physical structure of the SEM photograph of the PP fabric occurred. The original filaments were entirely covered with grafted polymers in the SEM image. Again, a significant change in the SEM images of the AN-g-PP is observed compared to that of the original PP which provides further physical evidence of grafting of AN onto the waste PP fabric. The difference in the photos of AN-g-PP and amidoximated AN-g-PP indicates the amidoximation of acrylonitrile grafted polypropylene fabric.
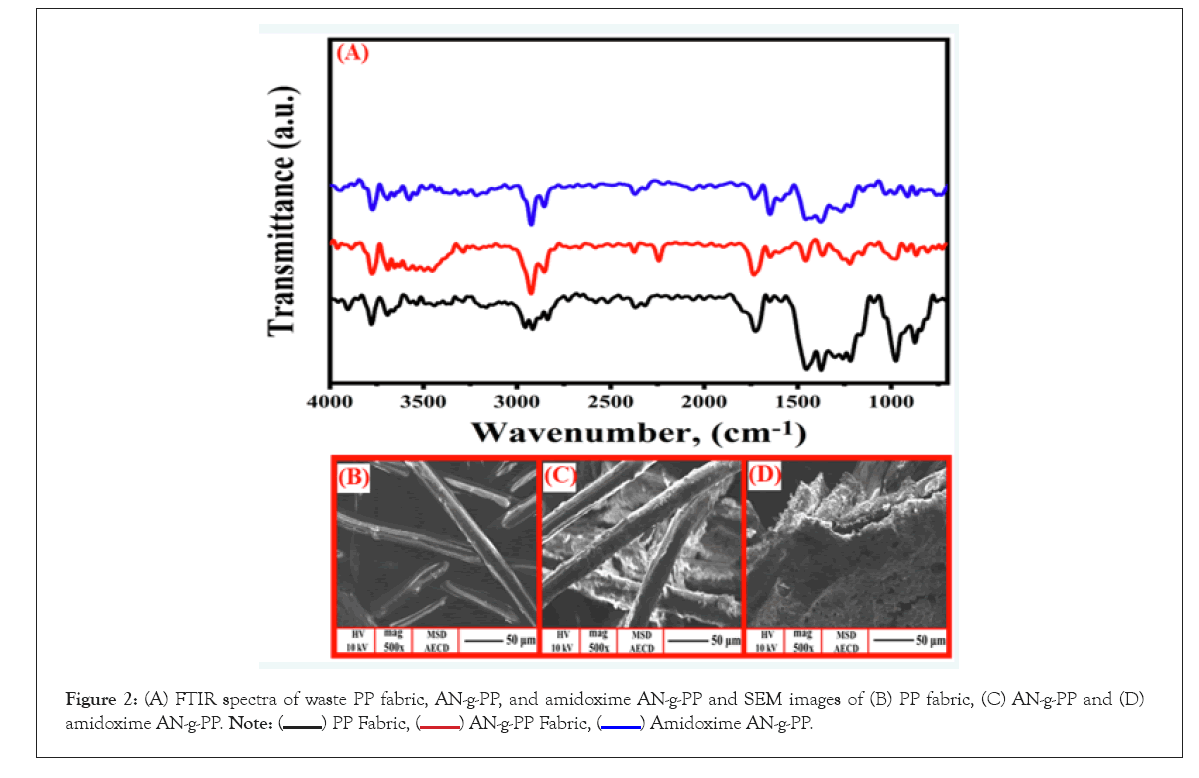
Figure 2: (A) FTIR spectra of waste PP fabric, AN-g-PP, and amidoxime AN-g-PP and SEM images of (B) PP fabric, (C) AN-g-PP and (D) amidoxime AN-g-PP.
Thermo-gravimetric analysis and dynamic mechanical analysis
Thermo-Gravimetric Analysis (TGA) of the original PP fabric, AN-g-PP fabric, and amidoximated AN-g-PP was performed to examine their thermal hardness. The results displayed in Figure 3A revealed that the thermal stability of the pristine PP was up to 227°C. Then, its weight loss kicks off and left 6.58% residues over 600°C. The weight loss appearing in the initial stage is due to moisture sucked up from the air. The AN-g-PP stands thermally persistent up to 230°C and then starts weight loss because of the degradation of its grafted chain and base polymer. It yields 28.64% residue upon heating over 600°C. The amidoximation of the AN-g-PP fabric results in the reduction of its thermal strength as its weight loss initiates at a very low temperature due to the complex decomposition of the grafted, amidoximated, and original PP chain. It generates 2.78% residue over 600°C. The stability of PP fabric at high temperatures indicates that its modified forms can be applied in the adsorption of heavy metals like Cr (VI). Dynamic mechanical analysis of waste PP fabric, AN-g-PP, and amidoximated AN-g-PP are shown in Figure 3B. As observed, the modulus of AN-g-PP is smaller than that of the original PP fabric and after amidoximation of the AN-g-PP, it exhibits a significant increase. The initial modulus loss was attributed to the moisture present in the samples. The storage modulus of PP fabric and AN-g-PP shows a gradual decrease with the increase of temperature and become zero at around 155°C. On the other hand modulus of amidoximated AN-g-PP increases initially and after going through a certain maximum value its decreases continuously and reaches zero at 173°C. The glass transition region for the original PP fabric begins at around 40°C. The glass transition states of AN-g-PP and amidoxime AN-g-PP initiate at around 47°C and 88°C respectively.
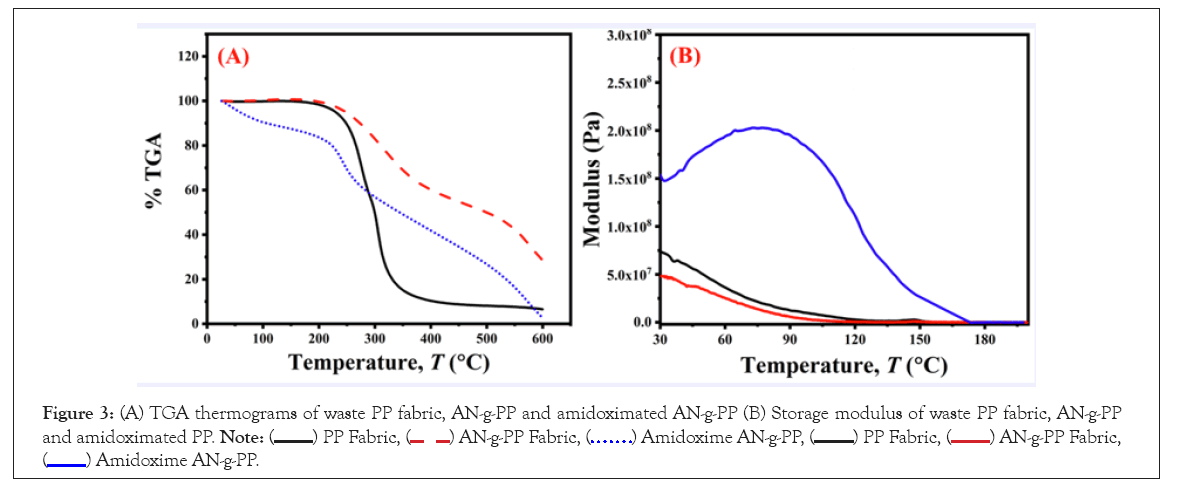
Figure 3: (A) TGA thermograms of waste PP fabric, AN-g-PP and amidoximated AN-g-PP (B) Storage modulus of waste PP fabric, AN-g-PP and amidoximated PP. 

Effect of initial Cr (VI) concentration and study of adsorption isotherms
Figure 4A explains the change of adsorption capacity with the initial Cr (VI) concentration (129 ppm, 223 ppm, 309 ppm, and 373 ppm). The Qe value increases gradually when the concentration of metal ion rises which could be demonstrated by considering the chelating sites present on the adsorbent surface. With the augmentation of metal ion concentration, the rate of occupation of chelating sites by the Cr (VI) molecule increases which favor the adsorption process.
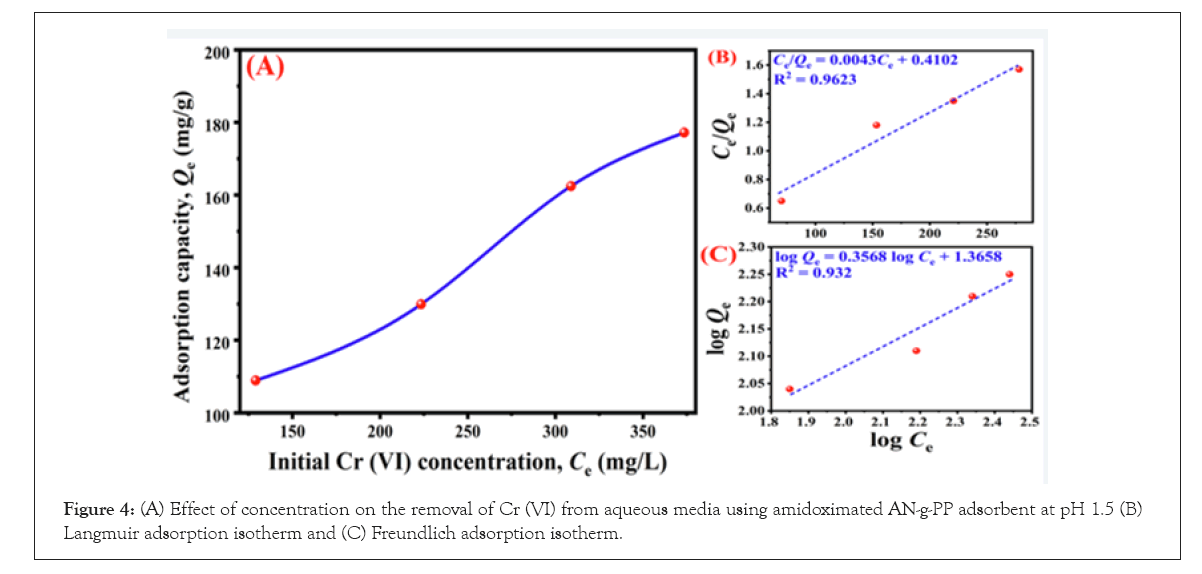
Figure 4: (A) Effect of concentration on the removal of Cr (VI) from aqueous media using amidoximated AN-g-PP adsorbent at pH 1.5 (B) Langmuir adsorption isotherm and (C) Freundlich adsorption isotherm.
However, at certain strength of metal ion, the adsorption sites will be saturated and then adsorption will be reached at a plateau value.
For the elucidation of equilibrium adsorption, the adsorption data were fitted to Langmuir and Freundlich isotherms [30]. The linear forms of the isotherms are given by the equations (5) and (7).
Langmuir:


Freundlich:

Where, Ce, Qe, and Qo are the initial Cr (VI) concentration (mg/L), equilibrium, and monolayer adsorption capacity (mg/g) respectively. b indicates the affinity of the binding sites and adsorption energy. The equilibrium parameter, RL describes the favorability of an adsorption process such as for a favorable adsorption process, 0<RL<1; RL=implies linear adsorption; RL 1 and 0 indicate unfavorable and irreversible adsorption, respectively [31]. The value of Qo and b can be obtained from plot Ce/Qe vs. Ce as shown in Figure 4B and listed in Table 1. The Qo value was found to be 232.56 mg/g.
| Langmuir | Cr (VI) |
|---|---|
| Qo (mg/g) | 232.56 |
| RL | 0.29 |
| R2 | 0.9623 |
| Freundlich | Cr (VI) |
| KF (mg/g) | 23.22 |
| n (L/mg) | 2.8 |
| R2 | 0.932 |
Table 1: Parameters of different adsorption isotherms.
The RL value calculated from equation 6 was obtained at 0.29 which lies between 0 and 1 indicating favorable Cr (VI) adsorption by the amidoximated AN-g-PP fabric. The correlation coefficient, R2for Langmuir adsorption was 0.9623. Equation 7 exhibits the linear representation of the Freundlich isotherm (Figure 4C). The magnitude of heterogeneity factor n and Freundlich constant KF can be estimated from the slope and the intercept of the plot log Qe against log Ce. For favorable adsorption, n is greater than one [31]. In this study, the n value obtained was 2.80. Thus the adsorption of Cr (VI) onto the amidoximated AN-g-PP is a favorable process. Then the regression coefficient, R2 for Freundlich isotherm was 0.9320.
Considering all the isotherm parameters it can be concluded that the experimental sorption data follows Langmuir as well as Freundlich isotherm models.
Effect of contact time on the removal of Cr (VI) ions and kinetic study
To investigate the effect of the amidoximated AN-g-PP was placed in an aqueous solution of Cr (VI) at pH 1.5 and an initial metal concentration of 120 ppm. The change of concentration was monitored at regular intervals shown in Figure 5A. As observed, the initial sorption of Cr (VI) was fast and eventually comes to the plateau after 40 hours with the highest adsorption of 126.82 mg/g.
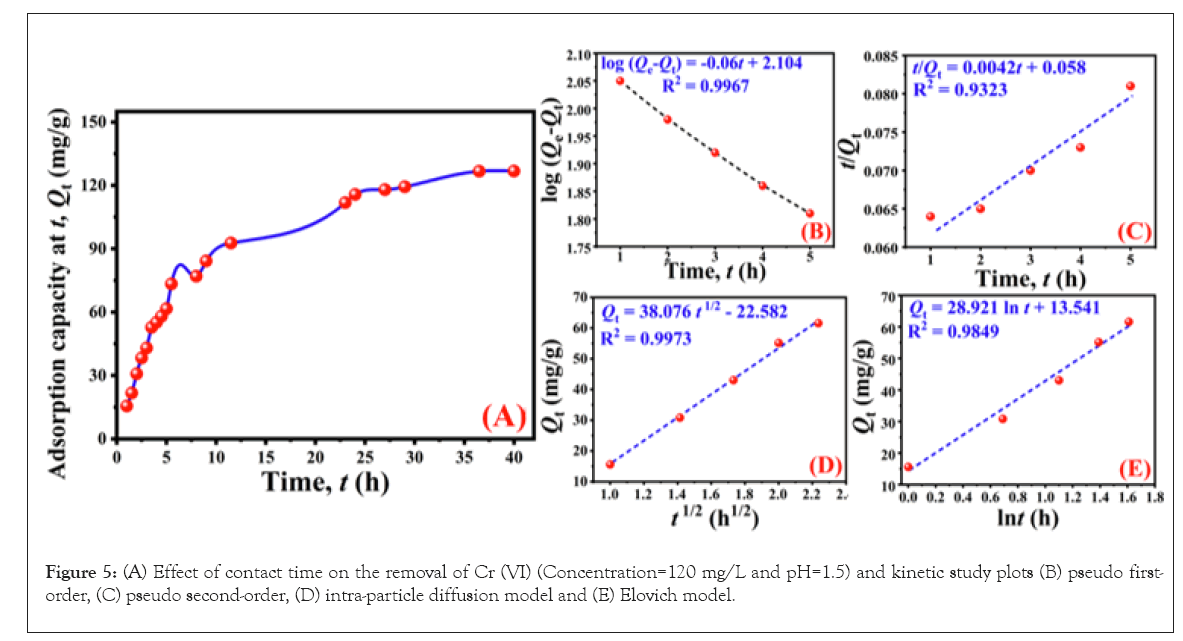
Figure 5: (A) Effect of contact time on the removal of Cr (VI) (Concentration=120 mg/L and pH=1.5) and kinetic study plots (B) pseudo first-order, (C) pseudo second-order, (D) intra-particle diffusion model and (E) Elovich model.
The kinetic data of Cr (VI) adsorption were fitted to several kinetic models including the first-order kinetic model, second-order kinetic model, intra-particle diffusion model, and Elovich model [32]. The linearized forms of the models are shown by the following equations:
First order kinetic model:
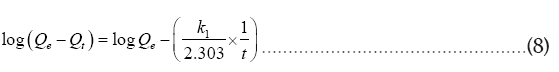
Second order kinetic model:
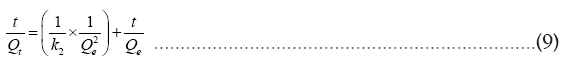
Intra-particle diffusion model:

Elovich model:

Where Qe and Qt are the adsorption capacity at equilibrium and at time t respectively in mg/g of the adsorbent. k1 (1/h), k2 (g/h. mg) and ki (mg/g.h)1/2 represent rate constant of the first order, second order, intra-particle diffusion respectively. In Elovich kinetic model, “ α ” indicates the initial adsorption rate constant, and “ β ” describes the desorption rate constant. The kinetic parameters for the adsorption of Cr (VI) are given in Table 2.
| Pseudo first-order | Cr (VI) |
|---|---|
| k1 (1/h) | 0.138 |
| Qe (mg/g) | 127.06 |
| R2 | 0.9967 |
| Pseudo second-order | Cr (VI) |
| k2 (g/h. mg) | 0.0003 |
| Qe | 238.1 |
| R2 | 0.9323 |
| Intra-particle diffusion | Cr (VI) |
| ki (mg/g. h) | 38.076 |
| C | –22.582 |
| R2 | 0.9973 |
| Elovich model | Cr (VI) |
| a | 13.541 |
| b | 28.921 |
| R2 | 0.9849 |
Table 2: Kinetic parameters for the adsorption of Cr (VI) onto the amidoximated AN-g-PP adsorbent.
As seen from the first-order kinetic model demonstrated in Figure 5B, the value of R2 was very high (˃0.99) and the calculated equilibrium adsorption capacity was 127.06 mg/g. Figure 5C interprets the second-order kinetic model. The calculated Qe value was 238.10 mg/g which is different the experimental Qe, also the correlation coefficient value, R2 is low. In the case2 of the intra-particle diffusion model, the magnitude of R was high but the line obtained from plotting Qt against t1/2 (Figure 5D) did not pass through the origin and thus intra-particle diffusion was not the rate-limiting step for the Cr (VI) removal using amidoximated AN-g-PP. The Elovich model (Figure 5E) exhibits smaller value of R2 compared to other models.
Therefore, we conclude that owing to the larger value of correlation coefficient in contrast to the other models and excellent consensus between the experimental (126.82 mg/g) and calculated (127.06 mg/g) equilibrium adsorption capacity, the adsorption of Cr (VI) onto the amidoximated AN-g-PP adsorbent follows the mechanism of the pseudo-first-order kinetic model.
Effect of pH and thermodynamic study
The adsorption capacity of an adsorbent largely depends on the pH of the medium. The change of pH affects the ionization of acidic and basic groups of the adsorbent due to their protonation and de-protonation, hence the surface structure of the adsorbent could be modified. Also, Cr (VI) remains in different forms when solution pH changes. Figure 6A represents the effect of pH on the adsorption of Cr (VI) ions onto the amidoximated AN-g-PP adsorbent. It is noticed that with the decrease of pH of the solution, the elimination of the metal ions increases gradually and the maximum sorption obtained was 126.82 mg/g at pH 1.5. A similar trend of Cr (VI) adsorption was found by Nazia Rahman [27]. The dissimilarity of the removal of Cr (VI) by the aforementioned adsorbent can be explained by the fact that at different pH, the adsorbent is attracted by the different species of the metal ion, such as at acidic pH, these forms are H2CrO4, HCrO4–, CrO42–, Cr2O72–. In addition, at low pH, the amino groups (–NH2) that exist on the surface of the amidoximated adsorbent become protonated and form positively charged, –NH3+ that attract the species of the metal ions which are negatively charged because of the coulomb forces. Conversely, at a high pH value, the –NH2 groups are de-protonated by the formation of negatively charged –NH2...OH–. The repulsive force operating between –NH2...OH– and negatively charged Cr (VI) ions are responsible for the reduction of adsorption of the metal ions.
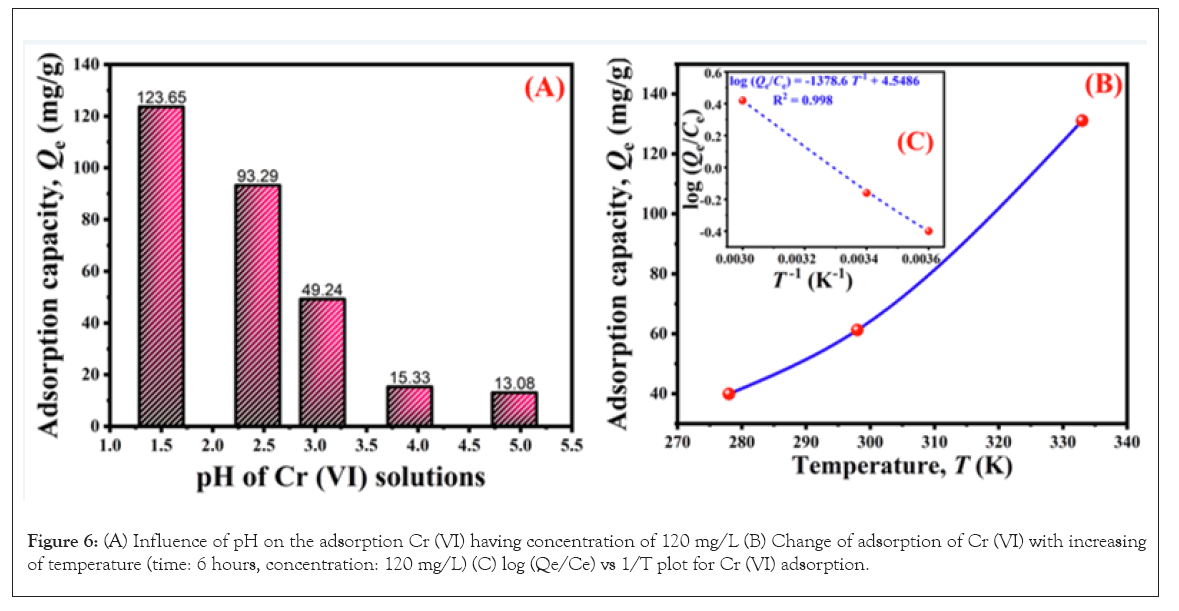
Figure 6: (A) Influence of pH on the adsorption Cr (VI) having concentration of 120 mg/L (B) Change of adsorption of Cr (VI) with increasing of temperature (time: 6 hours, concentration: 120 mg/L) (C) log (Qe/Ce) vs 1/T plot for Cr (VI) adsorption.
The adsorption of Cr (VI) using amidoximated AN-g-PP was studied at different temperatures as shown in Figure 6B. The Van’t Hoff equation for free energy change is as follows:


Where, ΔG0 is the standard free energy change in KJ/mol,ΔS0 and ΔH0 represent the standard entropy change in KJ/mol and the standard enthalpy change in kJ/mol/K respectively, Kd is a constant at a certain temperature, the molar gas constant is R in J/mol/K and T indicates the Kelvin temperature in K. The value of ΔS0 and ΔH could be calculated from the intercept and slope of Figure 6C, and different thermodynamic parameters are given in Table 3. As observed from the table, the adsorption of Cr (VI) was spontaneous and thermodynamically reasonable as the value of ΔG0 was negative at different temperatures. At higher temperatures, the sorption efficiency increases as shown in Figure 6B. Similar Cr (VI) adsorption change with temperature was observed by Nazia Rahman [33]. The positive value of ΔH0 (26.40 J/mol/K) indicates the adsorption process was endothermic. The removal of Cr (VI) utilizing the aforementioned adsorbent went on by the increase of disorder and degree of freedom at the solid-liquid interface due to the positive value of ΔS0 (0.087 kJ/mol) [34].
| Temperature (K) | ΔG0 (kJ/mol) | ΔH0(kJ/mol) | ΔS 0(J/mol/K) | R2 |
|---|---|---|---|---|
| 278 | –23.71 | 26.4 | 0.087 | 0.998 |
| 298 | –25.45 | |||
| 333 | –28.5 |
Table 3: Thermodynamic parameters for the sorption of Cr (VI).
Desorption study
The adsorbed Cr (VI) ions were desorbed from the amidoxime adsorbent surface for the reuse of the adsorbent. The desorption process was performed by treating the adsorbent with 2M NaOH solution for 24 hours. The desorption ratio obtained was 80%.
The adsorbent is essential for the removal of Cr (VI) as compared to the other adsorbent listed in Table 4.
| Adsorbent | Adsorption capacity (mg/g) |
|---|---|
| Amidoximated AN grafted waste PP (present study) | 232.56 |
| Amidoximated-PE [27] | 200 |
| Activated carbon derived from acrylonitrile-divinyl benzene Co- polymer [35] | 101.2 |
| Amine functionalized nanofibers [29] | 137 |
| Polypyrole/Fe3O4 magnetic nanocomposite [36] | 243.9 |
| Surface modified tannery waste [37] | 217 |
Table 4: Comparison of maximum adsorbent capacity of various adsorbents.
Radiation-induced grafting method was applied for the preparation of the AN-g-PP from the reaction between waste PP and 60% AN at 80°C for 4 hours. At 30 kGy radiation dose, the graft yield was 150% using H2SO4 as an additive. To obtain an amidoximated adsorbent, the AN-g-PP was allowed to react with hydroxylamine hydrochloride. The adsorbent was inspected using SEM, TGA, FTIR, and DMA and applied for the removal of Cr (VI) from the aqueous solution. Kinetic data were better fitted to the pseudo-first-order model maintaining a good consensus of experimental and theoretical adsorption capacity and equilibrium adsorption data were explained using both Langmuir and Freundlich isotherm model. The removal process was endothermic and spontaneous as seen from the thermodynamic investigation. The result of desorption and reuse of the amidoximated adsorbent was also satisfactory. Thus, it can be decided that the adsorbent is suitable for the removal of Cr (VI) from the aquatic environment because of its high removal capacity, low cost, and widely available.
The authors would like to thank the technical support of the IAEA (International Atomic Energy Agency) to carry out the study. We are also grateful to the Gamma Source Division of the Institute of Food and Radiation Biology, Atomic Energy Research Establishment.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
All data generated or analyzed during this study are included in this published article
Competing interests
The authors declare that they have no competing interests
[Crossref]
Citation: Sardar MN, Rahman N, Sultana S, Dafader NC (2022) Preparation of Amidoximated Acrylonitrile-g-Waste Polypropylene Adsorbent by Radiation Grafting Method and Its Application on the Adsorption of Cr (VI) from Aqueous Medium. J Thermodyn Catal. 13:307
Received: 22-Sep-2022, Manuscript No. JTC-22-19327; Editor assigned: 26-Sep-2022, Pre QC No. JTC-22-19327 (PQ); Reviewed: 10-Oct-2022, QC No. JTC-22-19327; Revised: 17-Oct-2022, Manuscript No. JTC-22-19327 (R); Published: 24-Oct-2022 , DOI: 10.37532/2157-7544.22.13.307
Copyright: © 2022 Sardar MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.