Journal of Food: Microbiology, Safety & Hygiene
Open Access
ISSN: 2476-2059
ISSN: 2476-2059
Research Article - (2016) Volume 1, Issue 2
This study was carried out to investigate the effect of sucrose solution of different concentrations on the overall quality of mango slices, packed in glass jars and stored at ambient temperature (30-35ºC) for 90 days. The treatment were T0 (mango slices + mango juice (control) T1 (mango slices + 20ºbrix sucrose solution) T2 (mango slices + 30%brix sucrose solution) T3 (mango slices + 40ºbrix sucrose solution) T4 (mango slices + 50ºbrix sucrose solution) T5 (mango slices + 60ºbrix sucrose solution). These samples were studied physicochemically for ascorbic acid content, %acidity, pH, total soluble solids (TSS), sugar-acid ratio, reducing sugar, non-reducing sugar and organolaptic evaluation. The ascorbic acid content decreased from 35.43 to 21.70 mg/100 g, Titratable acidity increased from 1.207 to 1.333%, pH decreased from 4.058 to 3.657, TSS increased from 26.60 to 31.50ºbrix, sugaracid ratio increased from 21.93 to 23.52, reducing sugar increased from 8.735 to 9.820%, non-reducing sugar decreased from 15.29 to 13.52%, during storage. In organoleptic evaluation, samples T2 and T3 were found the most acceptable to the panel. Statistical analysis showed that storage intervals and treatment had a significant (P<0.05) effect on physico–chemical and sensory analysis of mango slices.
Keywords: Mango slices; Quality; Ascorbic acid; Sucrose solution
Mango (Mangifera indica L.) family Anacardiaceae commonly called “King of fruits”, is native to Southern Asia, especially Burma and Eastern India. Mango is considered as fruit of excellence and thus has prominent position among commercial fruits grown in Pakistan. It is famous for its excellent flavor, attractive fragrance and nutritional value. Mango plays an important role in balancing the diet of human being by providing about 64-86 calories energy. Mango as an emerging tropical export crop is produced in about 90 countries in the world with a production of over 25.1 million tonnes. Asia is the main producer with 76.9% of the total world production, followed by America with 13.38%, Africa with 9% and less than 1% each for Europe and Oceania. Pakistan stands at 5th position among main mango producing countries with production of 938000 tones with a share of 7.6% in the world market [1]. The mango is one of the most cultivated tropical fruits in the World, with India, China and Mexico being the main producers. The fruit is an excellent source of fiber and vitamins A, C and the B complex, and has become more and more appreciated by consumers [2].
Mango is the second major fruit crop in Pakistan. At present it is grown on an area of 93.5 thousand hectares. Pakistan is blessed with many important leading commercial varieties of mango such as Sindhri, Langra, Anwar Ratol, Summer Bisht, Fajri, Fazali, Zafran, Saroli, Dusheri, Gulab Khas, Swarnarica, Bagan Pali, Chuansa Black & White, and Neelum [3]. Mango being a climacteric fruit possesses a very short shelf life and reach to respiration peak of ripening process on 3rd or 4th day after harvesting at ambient temperature [4]. The shelf life of mango varies among its varieties depending on storage conditions. It ranges from 4 to 8 days at room temperature and 2-3 weeks in cold storage at 13°C [5]. A difference among varieties exhibited 4 days of shelf life for Baneshan, Tomy Atkins and Kit [4,6] as compared to 8-9 days of Alphanso [7].
Due to mishandling, inadequate storage or lack of post-harvest technical knowledge producers and traders have to face about 20-30% losses [8] and loss of this perishable commodity is estimated up to 320.7 thousand tons annually with a value of Rs. 3.0 billion in the country [9]. Spoilage of mango due to stem end and Anthracnose, limits its storage potential and the shelf life is decided on the bases of spoilage (10%) during storage [4].
Most mangos are consumed fresh, but some non-fibrous pulpy mango varieties are used for processing. However, substantial quantities of mangoes are wasted because of poor post-harvest management and lack or appropriate facilities in developing countries. Therefore, the development and application of inexpensive preservation techniques to produce high quality and acceptance products of mango could be beneficial, allowing a better utilization of the fruit [10-13]. Mangoes are delicious, but require peeling, removal of flesh from the seed, and slicing before consumption [14]. Newer varieties developed through intensive breeding and bio technological techniques demand screening for value addition with various process technologies, including preservation of the fruit in slices form [15]. As mentioned above the mango slices were dipped in the sugar solution for preservation and to study the shelf life for three months at room temperature.
Mangoes were processed as commercial products with the high sugar content of 50-60º Brix in the final product. The syruping is a step up process [16]. Principally, the product is gradually sweetened by adjusting sugar solution. Because sugar is the main ingredient, the preserved mangoes taste sweet and provide energy. Therefore overconsumption may contribute to high risk of health problems including diabetes, obesity, and high blood glucose [17-19]. Three simultaneous mass transfer phenomena occurs: an important water flow from the product to the solution; a solute transfer from the solution to the product; and a minor transfer of product’s own solutes (sugars, organic acids, minerals and vitamins) to the concentrated solution [20]. Mango is a good source of vitamin A and C, TSS (Total Soluble Solids) and Minerals etc. It is also a medium source of carbohydrates as a ripe mango pulp contains 16.9% carbohydrate [21]. The vitamin C content determined for ripe Amini fruits, 11 mg/100 gm flesh, was about one half of the value reported previously; values of from 18.24 to 25.16 mg % have been recorded [22,23]. Ascorbic acid is widely distributed in fresh fruits and green leafy vegetables. In Nigeria, vegetables cannot be relied upon to supply ascorbic acid since almost all vegetables are thoroughly cooked before consumption, thereby destroying the heat-labile ascorbic acid content. However fruits are consumed raw, thereby conserving the ascorbic acid content. Although most fruits are sweet due to the sugar content, they supply very minimal energy and are associated with maintenance of health and prevention of diseases [24-26].
Mango is a very delicious fruit, due to its good taste, sweetness, flavour and firm yellowish-red skin and every age people like it very much throughout the world. The fruit can be preserved with different methods. In this research program mango slices was soaked in sucrose solution of various concentrations in glass jars. The product was stored in order to evaluate its shelf life, physicochemically and orgaoleptically.
Ripened and unsoft sindhri mangoes were obtained from the fruit market of Peshawar, Khyber Pakhtunkhuwa, Pakistan. Sindhri variety one of the most important varieties of mango was selected for this purpose like ripened and unsoft in order to prepare the samples for experiment.
Six different concentrations of sucrose were dissolved in sterilized distlled water and then used naturally preserved the mango slices in natural mango juice (T0), sucrose syrup of 200 brix (T1); sucrose syrup of 300 brix (T2); sugar syrup of 400 brix (T3); sugar syrup of 500 brix (T4); sugar syrup of 600 brix (T5).
Pre-processing
Fruits were selected accordingly to the appearance of the skin and texture and were thoroughly washed under the running tape water and manually peeled and cut into slices with stainless steel knife.
Storage
The sucrose solution was prepared with different degree Brix staring from 200 brix up to 600 brix, 3.5 gram citric acid was added to the sugar solution. The cut slices were put into the 250 ml pre-sterilized bottles and hot filled with the sucrose solution till the last treatment of 600 brix. Bottles were sealed properly and placed in boiling water for 30 minutes to complete the sterilization process. The research samples were placed at ambient temperature (25°C) to study the effect of storage on slices shelf life. The mango slices were analyzed for TSS (total soluble solids), PH, titratible acidity (TA), ascorbic acid, reducing sugars, non-reducing sugars, total sugar, sugar/acid ratio (AOAC methods 2000) and sensory evaluated for taste, flesh color, texture, and flavor at 15 days interval of three months storage.
Propose scheme of study
Treatments of the mango slices:
T0=Mango slices + Mango juice (control)
T1=Mango slices + 200 Brix Sucrose Solution
T2=Mango slices + 300 Brix Sucrose Solution
T3=Mango slices + 400 Brix Sucrose Solution
T4=Mango slices + 500 Brix Sucrose Solution
T5=Mango slices + 600 Brix Sucrose Solution
Ascorbic acid: Ascorbic acid was determined by the standard method as reported in AOAC [27].
Preparation and standardization of the dye solution
Fifty mg of 2, 6 dichlorophenol indophenols dye and 42 mg of sodium bicarbonate were weighed, dissolved in distilled water and volume was made up to 250 ml. 50 mg of standard ascorbic acid was taken in 50 ml of volumetric flask and the volume was made up with 0.4% oxalic acid. 2 ml of this ascorbic acid solution was titrated against dye solution until light pink color was obtained which persisted for 15 seconds.
Titration of the sample
Ten ml of the sample was taken in 100 ml of volumetric flask and volume was made up to the mark by adding 0.4% oxalic acid. 10 ml of prepared sample was taken in the flask and was titrated against dye until light pink color appeared, which persisted for 15 seconds. Three consecutive readings were taken for each sample. The ascorbic acid was calculated by using the following formula;
F = Factor from standardization= 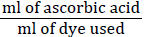
T = ml of dye used for sample
S = ml of diluted sample taken for titration
D = ml of sample taken for dilution
Titratable acidity
Titratable acidity was determined by the standard method of AOAC.
Standardization of the 0.1 N NaOH solution
About 6.3 g of oxalic acid was dissolved in distilled water and the volume was made to 1 litre with distilled water. About 4.5 g of NaOH pellets were taken and dissolved in distilled water and volume was made up to 1 litre. The burette was then filled with roughly prepared 0.1 N NaOH. 10 ml of 0.1 N oxalic acid was taken in a conical flask in triplicate. Two or three drops of phenolphthalein as indicator were added to each conical flask. The 0.1 N oxalic acid was titrated against 0.1 N NaOH solutions until pink light color was appeared, which persist for 15 seconds. Three consecutive readings were taken and the normality of NaOH was calculated using the formula:
N1 V1=N2 V2
Where
N1= Normality of oxalic acid.
V1= Volume of oxalic acid.
N2= Normality of NaOH
V2= Volume of NaOH.
Titration of samples
Ten ml of the sample was taken in 100 ml volumetric flask and diluted up to the mark. 10 ml of these samples were taken in a titration flask and add two or three drops of the phenolphthalein as indicator, then titrated against exact 0.1 N NaOH solution, until light pink color appeared, which persisted for 15 seconds. Three consecutive readings were taken and acidity was calculated by using the formula.
Where,
A= Sample taken for dilution
B= Sample taken for titration
pH
pH was determined by standard method of AOAC. For the determination of pH of samples, the pH meter was used. First it was standardized by using buffer solutions upto the pH (4 and 9) then 10 ml of sample was taken in a clean beaker and probe was directly dipped into the sample to record the pH value.
Total soluble solids
The Total Soluble Solids (TSS) was determined at room temperature by the recommended method of AOAC (2000) using ATAGO digital refractometer. The drop of representative sample was placed on the dry refractometer prism and readings were taken in “brix”, added the correction factor according to temperature.
Reducing sugar
Reducing sugar was determined by Lane and Eynon method as described in AOAC. (2000).
Reagents
Fehling A: Dissolved 34.65 g of CuSO4.5 H2O in 500 ml of distilled water. Fehling B: 173 g of potassium tartarate and 50 g of NaOH were taken in beaker. About 100 ml of water was added and dissolved the chemicals by stirring. The solution was transferred to 500 ml flask and volume was made up to the mark with distilled water. Methylene blue was used as indicator.
About 0.2 g of methylene blue was taken in a 100 ml volumetric flask and after dissolving in about 150 ml distilled water, the volume was made up to the mark by adding more distilled water.
Procedure
Ten ml of sample was taken in 100 ml volumetric flask and made up to the mark with distilled water. The burette was filled with this solution. Then 5 ml of Fehling A and 5 ml of Fehling B solution along with 10 ml distilled water was taken in a conical flask. The flask was heated until boiling without disturbing the flask. Sample solution was added from the burette drop by drop while boiling until the color became brick red in flask. A drop of methylene blue was added as indicator in the boiling solution of without shaking the flask. If color changes from red to blue for a moment, reduction isn’t complete and added more pulp solution till red color persists.
Calculations
5 ml of Fehling A + % ml of Fehling B = X ml of 10 % sample solution = 0.05 g of reducing sugar 100 ml of 10 % sample solution will contain
Non reducing sugars
Non reducing sugar was determined by Lane and Eynon method as described in AOAC (2000).
Procedure
Ten ml of the sample was taken in a volumetric flask and made the volume up to the mark with distilled water. 20 ml of this solution was taken in a flask and 10 ml of 1 N HCl was added, and then heated this solution for 5-10 minutes. After cooling 10 ml of 1 N NaOH was added and made this solution up to 250 ml.
This sample solution was taken in a burette. 5 ml Fehling A and 5 ml Fehling B solution along with 10 ml of distilled water was taken in a conical flask and boiled. When boiling started, it was titrated against the sample solution from the burette till changed to red-bricked color. It is tested with methylene blue as indicator till brick red color persisted.
Calculations
X ml of sample solution contains = 0.05 g if reducing sugar.
250 ml of sample contains =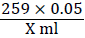 = Y g of reducing sugars
= Y g of reducing sugars
This 250 ml of sample solution was prepared from 20 ml of 10 %
Sample solution contains Y x 100/20 = P g reducing sugar
10 ml of sample solution contain = P g of reducing sugar
100 ml of sample solution contain = P x 100/10= Q g of total reducing sugar.
Q g of reducing sugar = inverted sugar + free reducing sugar.
Non-reducing sugar = total reducing sugar-free reducing sugar
Organoleptic evaluation
Selected samples of mango slices were evaluated organoleptically for color, flavor, and overall acceptability by the panels of 10 judges. The evaluation was carried out by using 9 points hedonic scale of [28].
Testing procedure
A panel of 10 judges selected of the relevant subject on the basis of their experience in sensory evaluation tests of food. The method involved was presenting the panel members with the sample and asking them to judge for color, flavor and overall acceptability. Panelists were requested to express their detection of their color and flavor by allotting the numbers from a scale (1-9) where 1 represented extremely dislike and 9 represented extremely liked.
Sensory evaluation
The following was used for grading the research samples showing grade scale from 1 to 9. scale: (9 like extremely) (8 like very much) (7 like moderately) (6 like slightly) (5 neither like nor dislike) (4 dislike slightly) (3 dislike very much) (2 dislike moderately) (1 dislike extremely).
Mango slices packed in glass jars stored at ambient temperature (30-35 Cº) and then physicochemically and organoleptically studied were conducted and the following results achieved during this experiment.
Ascorbic acid
The initial amount of ascorbic acid content of mango slices of sample T0 to T5 was 35.43 mg/100g, which was gradually decreased to 20.88, 21.84, 24.53, 23.97, 22.3, and 16.07 mg/100 g respectively during storage. The mean ascorbic acid content was significantly (P< 0.05) decreased from 35.43 mg/100 g to 21.70 mg/100 g during storage. For treatments maximum mean was observed in T3 (27.75) followed by T4 (26.69) mg/100 g, while minimum value was recorded in sample T5 (23.36) followed by T0 (24.30) mg/100 g. Maximum decrease was observed in sample T5 (54.64%) followed by T0 (41.06%), while minimum decrease was recorded in sample T2 (30.76%) followed by T3 (32.34%) (Table 1). This loss of ascorbic acid can be attributed to environmental factors like high temperature during sterilization and storage.
| Treatments | Storage interval (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | 90 | %Dec | Means | |
| T0 | 35.43 | 25.76 | 23.92 | 22.17 | 21.9 | 20.07 | 20.88 | 41.06 | 24.30cd |
| T1 | 35.43 | 26.58 | 25.54 | 24.5 | 23.91 | 22.15 | 21.84 | 38.35 | 25.71bc |
| T2 | 35.43 | 31.13 | 30.25 | 28.45 | 26.77 | 25.94 | 24.53 | 30.76 | 24.50cd |
| T3 | 35.43 | 30.98 | 27.86 | 27.15 | 25.47 | 24.4 | 23.97 | 32.34 | 27.75a |
| T4 | 35.43 | 29.58 | 26.41 | 26.06 | 24.2 | 22.25 | 22.93 | 35.28 | 6.69ab |
| T5 | 35.43 | 28.06 | 25.39 | 21.58 | 19.22 | 17.79 | 16.07 | 54.64 | 23.36d |
| Means | 35.43a | 27.85b | 25.73c | 23.99cd | 22.41de | 20.60f | 21.70ef | ||
Table 1: Analysis of ascorbic acid (mg/100 g) of mango slices packed in glass jars. Figures with different small letters are statistical different (P< 0.05). LSD at 5% level for storage interval = 1.798. LSD at 5% level for treatments = 1.665
The statistical analysis showed that storage intervals and treatments had significant (P< 0.05) effect on the ascorbic acid content of mango slices during storage (Appendix-I). However, samples with 30 0brix solution retained maximum ascorbic acid content during storage. These results are in agreement with the findings of [29] who reported the losses of ascorbic acid during storage. The losses of ascorbic acid in mango pulp had also been observed by [30].
Titratable acidity
Initially Titratable acidity of samples T0 to T5 was 1.20, 118, 1.25, 1.19, 1.19 and 1.23% respectively, which were gradually increased to 1.33, 1.32, 1.32, 1.25, 1.38 and 1.40% respectively during storage.
The mean for Titratable acidity significantly (P< 0.05) increased from 1.207% to 1.333% during storage. For treatments maximum mean values were recorded in sample T5 (1.313%) followed by T0 (1.290%), while minimum mean value was observed in sample T3 (1.197%) followed by T2 (1.253%). During storage maximum increase was observed in sample T4 (15.96%) followed by T5 (13.82%) while minimum increase was observed in sample T3 (4.8%) followed by T2 (5.30%) (Table 2). Statistical analysis showed that storage intervals and treatments had a significant effect (P< 0.05) on Titratable acidity of mango slices during storage (Appendix II). Similar results were obtained by [31], who reported an increase in Titratable acidity of mango pulp stored in glass bottles. This increase may be due to high storage temperature, formation of acidic compounds by degradation or oxidation of reducing sugars or break down of pectic bodies into acid as reported by [32].
| Treatments | Storage interval (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | 90 | % Inc | Means | |
| T0 | 1.2 | 1.24 | 1.31 | 1.31 | 1.32 | 1.32 | 1.33 | 10.83 | 1.290ab |
| T1 | 1.18 | 1.23 | 1.3 | 1.31 | 1.32 | 1.32 | 1.32 | 11.86 | 1.283abc |
| T2 | 1.25 | 1.19 | 1.2 | 1.24 | 1.27 | 1.3 | 1.32 | 5.6 | 1.253c |
| T3 | 1.19 | 1.19 | 1.18 | 1.2 | 1.22 | 1.15 | 1.25 | 5.04 | 1.197d |
| T4 | 1.19 | 1.2 | 1.23 | 1.24 | 1.29 | 1.33 | 1.38 | 15.96 | 1.266bc |
| T5 | 1.23 | 1.25 | 1.29 | 1.31 | 1.34 | 1.37 | 1.4 | 13.82 | 1.313a |
| Means | 1.207e | 1.217de | 1.252cd | 1.268bc | 1.293b | 1.298ab | 1.333a | ||
Table 2: Analysis of percent acidity of mango slices packed in glass jars. Figures with different small letters are statistically different (P< 0.05). LSD value at 5% level for storage interval = 0.03729. LSD value at 5% level for treatments = 0.03452
Organoleptic evaluation
The samples were sensory evaluated for color, flavor and overall acceptability at storage interval of 15 days for total period of 90 days by a panel of 10 judges experienced in organoleptic evaluation. The evaluation was carried out by using 9 point hedonic scale of [28].
Color
Initially the mean score of judges for color of mango slices samples (T0 to T5) was 9, which was decreased to 7.0, 4.8, 7.8, 7.9, 6.0, and 5.6 respectively during storage. The overall mean core of judges for color was significantly (P< 0.05) decreased from 9 to 6.517 during storage. For treatments maximum mean value was observed in sample T3 (8.200) followed by T2 (8.171), while minimum mean value was observed in sample T1 (6.214) followed by T5 (7.143). Maximum decrease was observed in sample T1 (46.66%) followed by T5 (37.77%), while minimum decrease was observed in T3 (12.22%) followed by T2 (13.33%) (Table 3).
| Treatments | Storage interval (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | 90 | %Dec | Means | |
| T0 | 9 | 8.6 | 8.2 | 7.8 | 7.8 | 7.5 | 7 | 22.22 | 7.986ab |
| T1 | 9 | 7.6 | 6.4 | 5.8 | 5.1 | 4.8 | 4.8 | 46.66 | 6.214d |
| T2 | 9 | 8.5 | 8.1 | 8 | 8 | 7.8 | 7.8 | 13.33 | 8.171a |
| T3 | 9 | 8.3 | 8.1 | 8.1 | 8.1 | 7.9 | 7.9 | 12.22 | 8.200a |
| T4 | 9 | 8.1 | 7.8 | 7.5 | 7 | 6.7 | 6 | 33.33 | 7.443bc |
| T5 | 9 | 8 | 7.7 | 7.1 | 6.5 | 6.1 | 5.6 | 37.77 | 7.143c |
| Means | 9.000a | 8.183b | 7.717bc | 7.383cd | 7.083de | 6.800de | 6.517e | ||
Table 3:Mean score of judges for color of mango slices packed in glass jars. Figures with different small letters are significantly different (P< 0.05). Each figure is the mean of observation of 10 judges. LSD value at 5%level for storage interval= 0.0.5943. LSD value at 5% level for treatments=0.5502
Statistical analysis showed that storage intervals & treatment ad a significant (P< 0.05) effect on the mean score of color of all mango slices samples during storage (Appendix VIII). These results are in agreement with the findings of [33] who recorded similar results in mango pulp. The reduction in color scores might be due to Millard reaction accelerated during storage.
Overall acceptability
Initially the mean score of judges for flavor of mango slices samples (T0 to T5) was 8.1, 8.2, 8.9, 8.8, 8.6, and 8.5, respectively, which were decreased to 4.3, 6.0, 7.5, 7.2, 4.7, and 3.4, respectively during storage. The overall mean score of judges for color was significantly (P< 0.05) decreased from 8.517 to 5.517 during storage. For treatments maximum mean value was observed in sample T2 (8.100) followed by T3 (8.086), while minimum mean value was observed in sample T0 (5.686) followed by T5 (5.729). Maximum decrease was observed in sample T5 (60.0%) followed by T0 (46.91%), while minimum decrease was observed in T2 (15.73%) followed by T3 (18.18%) (Table 4).
| Treatments | Storage interval (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | 90 | %Dec | Means | |
| T0 | 8.1 | 6.8 | 5.5 | 5.2 | 5.1 | 4.8 | 4.3 | 46.91 | 5.686c |
| T1 | 8.2 | 7.5 | 7.2 | 6.9 | 6.4 | 6 | 6 | 26.82 | 6.886b |
| T2 | 8.9 | 8.5 | 8.3 | 8 | 7.9 | 7.6 | 7.5 | 15.73 | 8.100a |
| T3 | 8.8 | 8.6 | 8.2 | 8.1 | 8 | 7.7 | 7.2 | 18.18 | 8.086a |
| T4 | 8.6 | 7.2 | 6.8 | 6.4 | 5.9 | 5.1 | 4.7 | 45.34 | 6.386b |
| T5 | 8.5 | 7 | 6.4 | 5.5 | 5.1 | 4.2 | 3.4 | 60 | 5.729c |
| Means | 8.517a | 7.600b | 7.067bc | 6.683cd | 6.400de | 5.900ef | 5.517f | ||
Table 4: Overall acceptability of mango slices packed in glass jars. Figures with different small letters are significantly different (P< 0.05). Each figure is the mean of observation of 10 judges. LSD value at 5%level for storage interval= 0.6206. LSD value at 5% level for treatments = 0.5745
Statistical analysis showed that storage intervals and treatment add a significant (P< 0.05) effect on the mean score of color of all mango slices samples during storage (Appendix X).
In this research work mango slices were preserved with sucrose solution of different concentrations and stored in glass jars at ambient temperature (30-35ºC) for a period of three months. The product was studied at two weeks interval for physiochemical and sensory evaluation. Among these samples the sample T2 (20 0brix sucrose concentration) followed by T3 (30 0brix sucrose concentration) were found most acceptable during Physico-chemical and sensory evaluation.
Suggestions for further research work on this project are:
1. The similar study effect on the quality of mango slices should be extended to the last one year storage period.
2. The same sucrose concentrations on mango slices should be studied in the cans.
3. The effect of non-nutritive sweetener should be studied on mango slices packed in glass bottles.