
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2022)Volume 13, Issue 2
Background: Residual neuromuscular blockade is a major deterrent to use of neuromuscular blocking drugs during general anesthesia. It is associated with potentially fatal acute respiratory events like upper airway obstruction, aspiration, hypoxia and atelectasis. In this study, we set out to determine the prevalence and complications of residual neuromuscular blockade in our setting.
Methods: We conducted a multicenter prospective cohort study in three referral hospitals in Uganda from June 2019 to March 2020. We recruited 485 adult patients admitted to the PACU who had received a non-depolarizing neuromuscular blocking drug during surgery. Our primary outcome was the prevalence of residual neuromuscular blockade in the PACU, which was defined as having a train of four ratio <0.9 and the secondary outcomes were associated factors and complications of residual neuromuscular blockade.
Results: Residual neuromuscular blockade was detected in 160 (33%) patients and acute respiratory events were noted in 177 (36.5%) patients. Elderly patients (age ≥ 65) and those who received additional doses of neuromuscular blocking drugs were more likely to have residual neuromuscular blockade, OR 2.39 and 6.08 respectively. Use of neostigmine, ASA III physical status and surgeries lasting >90 minutes were protective against residual neuromuscular blockade, OR 0.43, 0.30 and 0.18 respectively. We found no correlation between residual neuromuscular blockade and obesity or use of long acting neuromuscular blocking drugs. Residual neuromuscular blockade was not associated with statistically significant increased risk of developing acute respiratory events or increased length of stay in the PACU.
Conclusion: The prevalence of residual neuromuscular blockade is high. The risk is higher among elderly patients and those who receive additional doses of neuromuscular blocking drugs Intraoperatively. Use of reversal agents like neostigmine and routine monitoring of perioperative neuromuscular blockade could go a long way towards reducing the risk of residual neuromuscular blockade and its complications.
Neuromuscular blockade; Residual neuromuscular blockade; Acute respiratory events
Neuromuscular Blocking Drugs (NMBDs) were introduced into clinical practice in 1942, by Harold Griffith and Enid Johnson who used a purified extract of curare to paralyze patients undergoing various abdominal surgeries [1]. These drugs have since been widely used in anesthesia to facilitate endotracheal intubation, aid mechanical ventilation, and improve surgical conditions [2]. Following the use of these drugs, Residual Neuromuscular Blockade (RNMB) in the immediate postoperative period can result in Adverse Respiratory Events (AREs) like upper airway obstruction, hypoxia, regurgitation, and aspiration [3–5].
Beecher and Todd were the first to identify complications resulting from use of NMBDs when they noted a six-fold increased risk of death among post-surgical patients who had received NMBDs intraoperative [6]. This led to a call for improved techniques of monitoring depth of neuromuscular blockade (NMB) [7]. Clinical signs like grip strength, five seconds head lift, ability to cough and take a vital capacity breath are now considered obsolete indicators of adequate recovery of neuromuscular function due to the low sensitivity and specificity [8–11]. These have since been replaced with subjective and objective monitoring using a Peripheral Nerve Stimulator (PNS) [12,13]. Despite these advances, RNMB remains a common occurrence in the immediate postoperative period partly because it is greatly underestimated by anesthesia providers globally [14,15].
In Low and Middle-Income Countries (LMICs) like Uganda, it is neither routine nor mandated to monitor neuromuscular blockade and reversal agents are not regularly available. There is also a paucity of data from resource-constrained settings describing prevalence, associated factors, and complications of RNMB [16, 17]. We hypothesized that given the lack of appropriate equipment for monitoring depth of NMB and poor monitoring environments, the prevalence of RNMB and its associated complications would be high.
We conducted a multicenter prospective cohort study from 26th June 2019 to 31st March 2020 at three referral teaching hospitals in Uganda. Ethical approval was obtained from the School of Medicine Research Ethics Committee (SOMREC) of Makerere University (#REF 2019-095) and all methods were performed in accordance with the relevant guidelines and regulations. During the pre-operative period, verbal informed consent was obtained from all adult patients explaining that an accelerometer would be used as part of the monitors during the post-anesthesia period.
All adult (≥18 years) post-surgical patients admitted to the Post Anesthesia Care Unit (PACU) who had received a No Depolarizing Neuromuscular Blocking Drug (ND-NMBD) intraoperatively were assessed for eligibility to participate in the study and written deferred consent was obtained to allow use of their information in this study. We excluded patients with known neuromuscular disorders, patients allergic to the surface electrode adhesive, those admitted to the Intensive Care Unit (ICU) for respiratory support following surgery, patients who underwent procedures that prevented access to the forearm where electrodes would be placed and in case of failure to obtain the TOF ratio within five minutes of admission to the PACU.
From the anesthesia chart, we obtained patients’ demographic data such as; Age, type and duration of surgery, ASA physical status, NMBD used (type, re-dosing and total dose), technique used to monitor depth of NMB intraoperatively and, whether or not a reversal agent was used.
Upon admission into the PACU, a trained PACU nurse instituted routine patient care as per the hospital protocals. The skin over the course of the ulnar nerve was cleaned and degreased with isopropyl alcohol swabs. The Ag/AgCl hydrogel adhesive backed electrodes (EF Medical SRL, Italy) were placed appropriately, the cathode (black electrode) was placed distal and the anode (red electrode) proximal not more than five cm apart. The piezo-electric accelerometer was positioned on the distal ventral part of the thumb on the flexor side as shown in Figure 1.
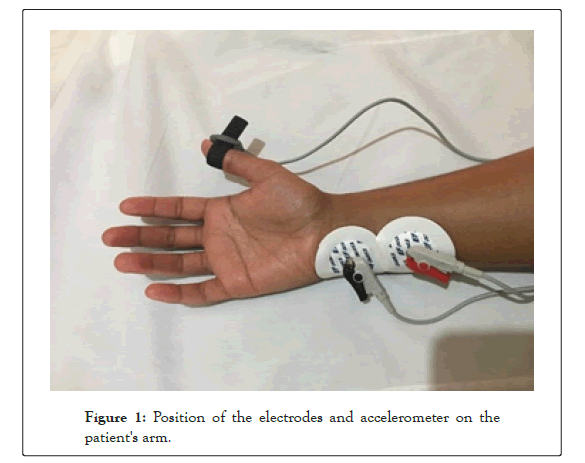
Figure 1: Position of the electrodes and accelerometer on the patient's arm.
A supramaximal TOF stimulus at a current of 50 MA, 0.2 ms duration at a frequency of 2 HZ was then applied via the surface electrodes and the evoked response at the thumb measured using an accelerometer (XAVANT STIMPOD NMS 450, South Africa). We obtained two consecutive TOF measurements 15 seconds apart, and the average of the two values recorded. If the measurements differed by more than 10%, an additional TOF stimulation was administered and the closest two ratios averaged. Residual neuromuscular blockade was defined as having a TOF ratio <0.9. These patients were then classified into moderate RNMB (TOF ratio between 0.7 and 0.89) and severe RNMB (TOF ratio <0.7) Patients with a TOF ratio <0.9 had repeated TOF stimulation every 10 minutes until a TOF ratio above 0.9 was achieved.
We used Murphy’s criteria shown in Table 1 to identify and classify AREs within 15 minutes of PACU admission, or at time of discharge, whichever came first. For the patients that stayed in the PACU for >15 minutes continued monitoring was done until discharge time and any new AREs were recorded. When an ARE was identified, the anesthesia provider was notified and management instituted as per the hospital protocols. The patient was deemed fit for discharge from the PACU and the study when he/she achieved an Aldrete score >eight.
| Grade 1: | Upper airway obstruction requiring an intervention (jaw thrust, oral, or nasal pharyngeal airway device placement) |
| Grade 2: | Mild-moderate hypoxemia [SpO2 of 90%–93%] on 3 L/min of O2 via nasal prongs that was not improved after active interventions (increasing O2 flows to >3 L/min, application of high-flow face mask O2, verbal requests to breathe deeply, tactile stimulation). |
| Grade 3: | Severe hypoxemia (SpO2 <90%) on 3 L/min of O2 via nasal prongs that was not improved after active interventions (increasing O2 flows to >3 L/min, application of high-flow face mask O2, verbal requests to breathe deeply, tactile stimulation). |
| Grade 4: | Signs of respiratory distress or impending ventilatory failure (respiratory rate >20 breaths per minute, accessory muscle use, tracheal tug). |
| Grade 5: | Inability to breathe deeply when requested by the PACU nurse, in a conscious patient. |
| Grade 6: | Patients complaining of symptoms of respiratory or upper airway muscle weakness (difficulty breathing, swallowing, or speaking). |
| Grade 7: | Patients requiring Intermittent Positive Pressure Ventilation (IPPV) using bag-mask ventilation or reintubation in the PACU. |
| Grade 8: | Clinical evidence or suspicion of pulmonary aspiration after tracheal extubation (gastric contents observed in the oropharynx and hypoxemia). |
PACU: Post anaesthesia care unit
IPPV: Intermittent positive pressure ventilation
Table 1: Murphy’s criteria for identifying acute respiratory events.
Statistical analysis
All analysis was performed using Stata version 13.1. The primary outcome of this study was prevalence of RNMB among post-surgical patients in Uganda and the sample size was determined based on a study by Neil, et al. [16]. The prevalence of RNMB and incidence of AREs were reported as percentages with 95% confidence intervals (C.Is). Postoperative RNMB was further classified as moderate (TOF ratio 0.7 to 0.89) or severe RNMB (TOF ratio <0.7).
Continuous variables were summarized as means and standard deviation (SD) or median and interquartile range [IQR]. Means were compared using the Student t test and medians were compared using the Mann-Whitney U test/Wilcoxon rank sum test.
Categorical variables were compared using the chi square tests or Fisher exact tests. Bar graphs, where appropriate, were drawn for data visualization.
The relationship between RNMB and potential explanatory variables was determined using a logistic regression model. Bivariate analysis was performed to determine any association between independent variables and the dependent variable like Age, BMI, ASA classification, length of surgery, type of ND-NMBD used and use of a reversal agent. Candidate variables with P values less than 0.25 were selected for multivariate analysis.
Confounding was assessed by considering a 10% change in the odds ratios of a model with the variable and one without the variable. Interaction was assessed on the multiplicative scale using the chunk test by forming two-way product terms between the variables after running a stepwise logistic model.
Results were presented in terms of p-values, unadjusted and adjusted odds ratios with their 95% confidence intervals. Variables with a p value <0.05 were considered significant. The goodness of fit of the final model was assessed using the Hosmer-Lemeshow test.
Patient characteristics
We enrolled 486 patients from three study sites and analysed 485 patients. One patient was excluded since the TOF ratio could not be obtained at the forearm due to technical difficulty, he had Open reduction and internal fixation of bilateral radial fractures and we could not access the forearm.
Baseline characteristics
Baseline demographics are shown in Table 2 comparing patients with RNMB and those with adequate recovery of neuromuscular function. The patient characteristics were mostly similar except; duration of surgery, type of NMBD used and the point at which the NMBD was administered p values <0.001, 0.013, 0.016 respectively (Table 2).
| CHARACTERISTICS | ALL | No RNMB (TOF ratio >90) | RNMB (TOF ratio <90) | P value |
|---|---|---|---|---|
| AGE (years) | ||||
| Median (IQR) | 40,28-52 | 39 (28-51) | 41 (28-60) | 0.113 |
| BMI, n (%) | ||||
| Normal (18.5-24.9) | 201 (46.6) | 129 (44.3) | 72 (51.4) | |
| <18.5 | 36 (8.4) | 26 (8.9) | 10 (7.1) | |
| 24.5-29.9 | 128 (29.7) | 94 (32.3) | 34 (24.3) | |
| 30.0 and above | 66 (15.3) | 42 (14.4) | 24 (17.1) | 0.345 |
| Type of surgery, n (%) | ||||
| Emergency | 104 (21.4) | 255 (78.5) | 126 (78.8) | |
| Elective | 381 (78.6) | 70 (21.5) | 34 (21.2) | 0.754 |
| ASA classification, n (%) | ||||
| I | 185 (38.1) | 114 (35.1) | 71 (44.4) | |
| II | 217 (44.7) | 148 (45.5) | 69 (43.1) | |
| III | 69 (14.2) | 54 (16.6) | 15 (9.4) | |
| IV | 14 (3.0) | 9 (2.8) | 5 (9.1) | 0.123 |
| Duration of surgery, n (%) | ||||
| 0-90 | 236 (48.7) | 139 (42.8) | 97 (60.6) | |
| >90 minutes | 249 (51.3) | 186 (57.2) | 63 (39.4) | <0.001 |
| Use of ND-NMBDs, n (%) | ||||
| Induction only | 88 (18.1) | 70 (21.5) | 18 (11.3) | |
| Maintenance only | 321 (66.2) | 211 (64.9) | 110 (68.7) | |
| Induction + maintenance | 76 (15.7) | 44 (13.5) | 32 (20.0) | 0.013 |
| ND-NMBDs used n (%) | ||||
| Cisatracurium | 162 (33.4) | 110 (33.9) | 52 (32.5) | |
| Rocuronium | 226 (46.6) | 162 (49.9) | 64 (40.0) | |
| Pancuronium | 47 (9.7) | 23 (7.1) | 24 (15.0) | |
| Atracurium | 50 (10.3) | 30 (9.2) | 20 (12.5) | 0.016 |
| Reversal with Neostigmine/Atropine, n(%) | ||||
| Yes | 393 (81.4) | 268 (83.0) | 115 (77.7) | |
| No | 90 (18.6) | 55 (17.0) | 33 (22.3) | 0.173 |
| Duration in the PACU (mins), median (IQR) | 15,10-25 | 13 (10-20) | 25 (17-35) | <0.001 |
| Aldrete score at PACU admission mean ± SD | 7.08 ±1.8 | 7.59 ± 1.7 | 6.06 ± 1.7 | <0.001 |
BMI: Body mass index
IQR: Interquartile range
ND-NMBDs: non-depolarizing neuromuscular blocking drugs
PACU: Post anaesthesia care unit
SD: Standard deviation
TOF: Train of four
Table 2: Baseline variables of patients stratified according to TOF ratio at admission to the post anesthesia unit.
Prevalence of residual neuromuscular blockade
Post-operative RNMB was noted in 160 (33%) patients, 79 (16.3%) had moderate RNMB and 81 (16.7%) had severe RNMB as shown in Table 3. Of the 81 patients with severe RNMB, 12 had no TOFR since they had a TOF count less than four (Table 3).
| Characteristic | All N (%) |
|---|---|
| TOF ratio >0.9 | 325 (67) |
| TOF ratio 70-89 (Moderate RNMB) | 79 (16.3) |
| TOF ratio <70 (Severe RNMB) | 81 (16.7) |
RNMB: Residual neuromuscular blockade
TOF: Train of four
Table 3: Train of four ratios on admission to the post anaesthesia care unit.
Factors associated with Residual neuromuscular blockade
Patients who received additional doses of NMBDs intraoperatively and the elderly patients (Age ≥ 65) were more likely to have RNMB on admission to the PACU, OR 6.08 (95% CI, 3.40-10.9) and 2.39 (95% CI, 1.23-4.66) respectively.
Surgeries lasting >90 minutes, use of neostigmine and ASA III physical status were protective against RNMB OR 0.18 (95% CI, 0.10-0.33), OR 0.43 (95% CI, 0.24-0.77) and OR 0.3 (95% CI 0.14-0.63) respectively, as shown in Table 4.
| Characteristic | Unadjusted OR, 95% CI | P-value | Adjusted OR, 95%CI | P-value |
|---|---|---|---|---|
| Age ≥ 65 | 2.76(1.55-4.93) | 0.001 | 2.39(1.23-4.66) | 0.010 |
| Duration of surgery > 90 mins | 0.49(0.33-0.71) | <0.001 | 0.18(0.10-0.33) | <0.001 |
| Neostigmine used at reversal | 0.73(0.46-1.18) | 0.199 | 0.43(0.24-0.77) | 0.005 |
| Use of long-acting NMBDs | 2.31(1.26-4.25) | 0.007 | 1.83(0.88-3.80) | 0.103 |
| Re-dosing NMBDs | 2.63(1.76-3.94) | <0.001 | 6.08(3.40-10.9) | <0.001 |
| ASA III | 0.45(0.23-0.85) | 0.014 | 0.30(0.14-0.63) | 0.002 |
ASA: American society of anesthesiologist
CI: Confidence interval
NMBDs: Neuromuscular muscular blocking drugs
OR: Odds ratio
Table 4: Multivariate logistic regression for factors associated with Residual neuromuscular blockade.
There was no correlation between RNMB and use of long acting NMBDs OR 1.83 (95% CI, 0.88-3.80).
Adverse respiratory events
Acute respiratory events were noted in 177 (36.5%) patients, 64 (36.2%) of whom had more than one ARE. The commonest AREs identified were; upper airway obstruction 52 (29.2%) followed by mild hypoxemia 43 (24.3%), Severe hypoxemia 24 (13.6%), need for assisted ventilation using intermittent positive pressure ventilation (IPPV) 21 (11.9%), impending respiratory failure 18(10.2%), inability to breathe deeply in an awake patient 13 (7.3%) and signs of upper airway muscle weakness like temporary inability to swallow or speak 9 (5.1%). None of the patients had evidence of aspiration (grade 8 ARE).
As shown in Figure 2, all grades of AREs were more common among patients with RNMB apart from grade 2 (mild hypoxemia). However, following multivariate analysis, there was no statistically significant increase in risk of developing AREs among patients with severe RNMB or 1.92 (95% CI; 0.98-3.78 p =0.05) (Figure 2).
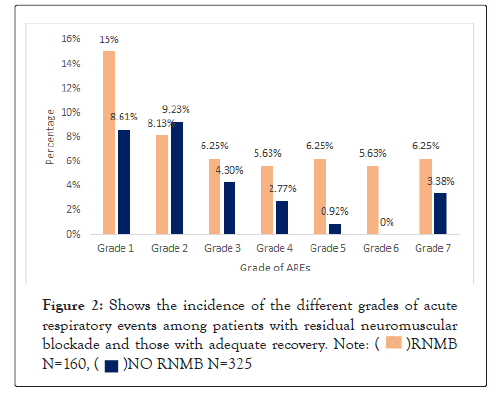
Figure 2: Shows the incidence of the different grades of acute respiratory events among patients with residual neuromuscular blockade and those with adequate recovery.
In this study, we set out to determine the prevalence, associated factors and complications of RNMB in our setting. The prevalence of RNMB was 33% and was more common among the elderly patients (age >65) and those who received additional doses of NMBDs. Factors protective against RNMB were; surgeries lasting >90 minutes, use of neostigmine and ASA III physical status. The incidence of AREs was 36.5% and the commonest AREs were upper airway obstruction and mild hypoxemia. Patients with RNMB did not have a statistically significant increased risk of developing AREs or increased duration of PACU admission.
The prevalence of RNMB noted in our cohort correlates with the average global prevalence, which ranges from 30%-50% as reported in a meta-analysis by Naguib and colleagues [17]. A similar study in sub-Saharan Africa carried out in a teaching hospital in Ethiopia found a much lower incidence of RNMB (12.9%), this could be because of the methodological differences [18]. In their study, RNMB was defined as having one twitch or fade following Double Burst Stimulation (DBS) at a current of 80 MA. There’s evidence suggesting that detection of fade at a TOFR >0.6 using DBS is not always possible and this could result in erroneously lower incidence of RNMB as patients with TOFR between 0.61 and 0.89 are missed [19].
Elderly patients were twice as likely to develop RNMB compared to those less than 65 years; similar findings were reported in a study by Murphy, et al. [20]. This could be explained by age-related muscle weakness and reduced physiologic function of the liver and kidneys which are responsible for metabolism and excretion of some of the ND-NMBDs [9, 21]. It is therefore advised to use routine monitoring and reversal agents whenever these drugs are used especially in a geriatric patient [20].
We also noted a six-fold increased risk of RNMB among patients who received an additional dose of ND-NMBDs intraoperatively. This is partly because none of the patients in our cohort was monitored using a PNS, so we postulate that the decision to administer additional doses of the ND-NMBDs was based on time since the last dose, clinical signs like return of spontaneous ventilation and surgeon’s request to administer additional doses. All these have been shown to be poor predictors of depth of NMB due to the high individual variability hence the general consensus to use subjective or objective monitoring [8, 21-28].
Neostigmine is currently the most studied anticholinesterase drug and it was the only reversal agent used in our study. There are mixed reports regarding its role in preventing RNMB, some studies show no benefit, while others report a lower incidence of RNMB whenever it is used [12, 29-31]. In the present study, it offered a 60% reduction in risk of RNMB. Additionally, we noted a lower incidence of RNMB among patients whose surgeries lasted >90 minutes, this is in agreement with previous studies [11, 32]. Long surgeries allow adequate time for spontaneous degradation of the NMBDs and a better chance of successful reversal with neostigmine when administered at shallow levels of NMB [33].
Globally, long-acting NMBDs are used less frequently by anesthesia providers because of the literature showing increased risk of RNMB whenever they are used [34]. In the present study, we did not find an increased risk of RNMB following use of the long-acting NDNMBDs. Possibly because only one tenth of the patients received the long acting NMBDs and they were mostly used for surgeries expected to last > 60 to 90 minutes.
The incidence of AREs in our cohort was twice higher than the average global incidence that ranges from 0.8%-19.7% [4, 32, 35]. This could be due to lack of standard monitoring intra-operatively, ill equipped and poorly staffed post anesthesia care units. As shown in Figure 2, there was a higher incidence of AREs among patients with RNMB. However, following multivariate analysis, we were unable to demonstrate a direct association between AREs and RNMB. This contradicts with findings from previous studies showing a strong association between AREs and RNMB [3, 4, 11]. Our findings are in line with results from a study by Stewart, et al. who also failed to show a direct association between AREs and RNMB following analysis for confounders like level of consciousness and temperature [32].
We did not collect information about core temperature and gender so we cannot account for the contributions from the above factors towards occurrence of residual neuromuscular blockade or acute respiratory events.
The strengths of the study are; the multicenter study design provides an overview of anesthesia practice across different settings
and the accelerometer used (Stimpod NMS 450) unlike the previous models, allows tri-axial monitoring which precludes the need to stabilize the patient's hand on a rigid board or align the accelerometer wafer with the direction of movement of the thumb. This makes it the ideal gadget for detecting RNMB especially among awake patients in the PACU.
The incidence of RNMB is high. It is more common among the elderly and those who receive additional doses of NMBDs. The risk is reduced when reversal agents like neostigmine are used and in surgeries lasting >90 minutes. Our study did not show a statistically significant association between RNMB and AREs or prolonged PACU admission time. However, given its potentially fatal complications, anesthesia providers should work towards curbing its occurrence through judicious use of NMBDs and reversal agents.
[Crossref] [Google Scholar] [pubmed]
[Crossref] [Google scholar] [pubmed]
[Crossref] [Google Scholar] [pubmed]
[Crossref] [pubmed] [Google Scholar]
[crossref] [Google Scholar] [pubmed]
[crossref] [Pubmed] [Google Scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[Crossref] [pubmed] [Google scholar]
[crossref] [pubmed] [Google scholar]
Citation: Emyedu A, Atumanya P, Okello E, Wabule A, Ssemogerere L, Mukisa J, et al. (2022) Prevalence and Complications of Residual Neuromuscular Blockade in the Post Anesthesia Care Units of Uganda. J AnesthClin. Res. 13:1045
Received: 24-Jan-2022, Manuscript No. JACR-22-15612; Editor assigned: 26-Jan-2022, Pre QC No. JACR-22-15612 (PQ); Reviewed: 09-Feb-2022, QC No. JACR-22-15612; Revised: 14-Feb-2022, Manuscript No. JACR-22-15612 (R); Published: 21-Feb-2022 , DOI: 10.35248/2155-6148.22.13.1045
Copyright: © 2022 Emyedu A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.