Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Article - (2023)Volume 3, Issue 3
Background: Urogenital mollicutes are a group of potentially pathogenic microorganisms that could be associated with serious complications related to reproductive failures and urogenital troubles. In Saudi Arabia, very little is known about the incidence of these pathogens. Here, we aim to investigate the prevalence of human urogenital mollicute infections caused by Mycoplasma hominis, M. fermentans, M. genitalium, Ureaplasma urealyticum and U. parvum in 100 female Saudi patients from Aseer region, suffering from gynecological and reproductive troubles.
Methods: Mollicutes detection was performed on vaginal swabs that were collected in 2018 using PCR amplification targeting 16S rRNA and species-specific genes in conjunction with culture.
Results: Overall, PCR detected mollicutes in the half of samples, unambiguously identifying M. fermentans (32%), M. hominis (11%), U. urealyticum (11%) and U. parvum (10%). Culture on specific medium was positive for 15 samples including M. fermentans (3%), M. hominis (11%) and Ureaplasma spp. (1%). U. urealyticum and U. parvum were found to be significantly associated with pregnancy complications and genital troubles (P<0.05). As for M. fermentans and M. hominis, no significant association was observed with any type of pathologies. However, for some urogenital/ gynecological troubles, odds ratio calculation showed that the occurrence risk is correlated to mollicute infections.
Conclusion: This study revealed a high prevalence of mollicute infections among female patients suffering from urogenital troubles, in Saudi Arabia, with a significant involvement of Ureaplasma species and an unexpected highrate of M. fermentans.
Urogenital mycoplasmas; PCR; Culture; Urogenitourinary troubles
With no cell wall and a minute size, mycoplasmas are considered as the smallest self-replicating procaryotes. These bacteria belong to the class of mollicutes and they are widely spread in nature. In humans, several mycoplasma species have been isolated from the urogenitourinary tract, the most frequent isolated species being Ureaplasma urealyticum, U. parvum, Mycoplasma hominis and M. genitalium. M. fermentans as well was isolated decades ago from the urogenital tract but much less frequently. Some of the urogenital mycoplasmas are commensals, while others have proved to be pathogens. Together, all these microorganisms form the unique and complex group of urogenital mycoplasmas, which has been often associated with a wide array of symptomatic and asymptomatic infectious diseases in the human urogenital tract in both males and females, including children [1].
In women, urogenital mycoplasmas may cause cervicitis, endometritis, tubal infertility, and Pelvic Inflammatory Disease (PID) resulting in damage to the fallopian tubes, which may lead to ectopic pregnancy. These bacteria can also cause adverse pregnancy outcomes such as abortion, preterm delivery, postpartum or post-abortal fever. They could also be the source of perinatal disorders, such as low birth weight and neonatal bacteremia/meningitis, which may lead to neonatal mortality and morbidity. Moreover, compelling evidence link the urogenital mycoplasmas, notably M. genitalium, with sexually transmitted infections. In the context of urinary tract, the pathogenic potential of mycoplasmas and ureaplasmas is not clearly described. Urethritis due to urogenital Mycoplasma species is less extensively described in women than in men [2].
For mycoplasmas diagnosis, culture has historically been the gold standard. Although, cultivation of these microorganisms has proved challenging because of their fastidious nature. Culture requires special, expensive, and complex media and it may take several days or weeks. Serology is more convenient and sensitive than culture, however, the use of serological assays is hampered by false-negative results resulting mainly from crossreactivity between Mycoplasma species. Nucleic acid-based tests, such as PCR, are devoid of these drawbacks. Molecular diagnostic techniques are fast, specific, and sensitive which explains their abundant use in laboratories for the detection of mycoplasmas. Recently, in Saudi Arabia, researchers and clinicians have started investigating urogenital Mycoplasma infections among female patients. Although, more studies are required in order to evaluate the potential association between this kind of bacterial infections and genital troubles, including infertility.
In the present study, 100 vaginal swabs collected from female patients presenting symptoms of different urogenital/ gynecological disorders, were examined for urogenital mollicutes using culture and PCR. The main purposes of this work are, (i) to evaluate the extent of urogenital mollicute infections, and (ii) to search for possible association between mollicutes and urogenital/gynecological troubles in the female Saudi population in Abha, the capital of Aseer region (Southwest of Saudi Arabia) [3].
Sample collection
The study population consisted of a total of 100 duplicate vaginal swabs collected from Saudi female patients in reproductive age (between 18 and 51 years old; mean age=30.22 years old) that have been referred to obstetrics and gynecology department at Abha maternity and children hospital (Abha town, Saudi Arabia). This study was approved by the ethical committee of the college of medicine-King Khaled university (Rec#2018-06-24) and all samples were taken from female volunteers, with personal information being kept confidential. Patients were divided into 4 groups: Women suffering from pregnancy complications (group 1), patients with genital pathologies (group 2), patients diagnosed with urological disorders (group 3), and infertility cases (group 4) (Table 1). All vaginal swabs were frozen at -80°C until they were forwarded to the mycoplasmas laboratory of Pasteur institute of Tunis, Tunisia [4].
| Disease | Number of women |
|---|---|
| Group N°1: Pregnancy complications | 45 |
| Group N°2: Genital disorders | 32 |
| Group N°3: Urogenital pathologies | 20 |
| Group N°4: Infertility | 6 |
Table 1: Clinical details of patients.
DNA extraction and PCR
Vaginal swabs that will be subject to PCR amplifications were immersed in SP4 medium upon receipt and were frozen at -80°C until their usage. Samples were prepared as described before with some modifications. Briefly, 100 μl of each sample was transferred to a microtube and centrifuged at 15000 rpm for 5 min at room temperature. After centrifugation, the supernatant was delicately discarded, the pellet was resuspended in 200 μl PBS buffer and centrifuged one more time at 15000 rpm for 5 min. Then, the pellet was resupended in 20 μl of PBS buffer. DNA was isolated by treatment with 80 μl of digestion buffer (10 mM Tris-HCl, pH 8.0; 0.45% Tween 20) and proteinase K (100 μg/ml). Lysates were heated for 1 h at 56°C, then for 10 min at 100°C, and centrifuged at 15000 rpm for 5 min. The supernatants were finally transferred to sterile microtubes and stored at -20°C. PCR was performed using specific primer pairs (Table 2). Genomic DNAs of all mycoplasma type strains were extracted and used as positive controls. Human DNA available in the laboratory was used as negative control [5].
| Mycoplasma species | Primer designation | Target gene | Amplicon size (bp) | Sequence (5’-3’) |
|---|---|---|---|---|
| M. hominis | Vaa_F | Vaa | 297 | GGAATAGCAACTACTGCTATTTTGC |
| Vaa_R | TTGATCAACTTTTTGTTGTTCACTTTTTGCGGAGGAATTTCAACTGGTGTCC | |||
| Mhp120’F | p120’ | 510 | CTGTTGTAATAGCATTTAAG | |
| Mhp120’R | CAATGGCTAATGCCGGATACGC | |||
| RNAH1 | 16S rRNA | 335 | GGTACCGTCAGTCTGCAAT | |
| RNAH2 | - | |||
| Ureaplasma spp. | UMS-125 | mba | 448 (U. urealyticum) 403 (U. parvum) |
GTATTTGCAATCTTTATATGTTTTCG |
| UMA-226 | CAGCTGATGTAAGTGCAGCATTAAATTC | |||
| U. urealyticum | Uu_16S rRNA_F | 16S rRNA | 313 | TAAATGTCGGCTCGAACGAG |
| Uu_16S rRNA_R | GCAGTATCGCTAGAAAAGCAAC | |||
| U. parvum | UPS1 | 16S rRNA | 678 | ATGAGAAGATGTAGAAAGTCGCTC |
| UPA | TTAGCTACAACACCGACCCATTC | |||
| M. fermentans | RW 005 | IS-like element | 205 | GGTTATTCGATTTCTAAATCGCCT |
| RW 004 | GGACTATTGTCTAAACAATTTCCC | |||
| Mf_16S rRNA_F | 16S rRNA | 611 | ACGGCCCACCAAGGCGATGATGTTT | |
| Mf_16S rRNA_R | GATCATTTAATGCGTTAGCTGCGC | |||
| M. genitalium | MgPa1_F | Adhesion protein | 281 | AGTTGATGAAACCTTAACCCCTTGG |
| MgPa3_R | CCGTTGAGGGGTTTTCCATTTTTGC | |||
| MG16-45F | 16S rRNA | 425 | TACATGCAAGTCGATCGGAAGTAGC | |
| MG16-447R | AAACTCCAGCCATTGCCTGCTAG |
Table 2: Characteristics of primers used in this study.
PCR was performed in a final volume of 50 μl including 5 μl of 10X Taq buffer, 1 μM MgCl2, 1.5 U of Taq polymerase (Sigma, Germany), 1 pmol of each primer, 0.2 mM dNTP (Sigma, Germany), and 7 μl of the extracted DNA. Amplification reactions were performed in a 2720 thermal cycler (Applied biosystems, USA). Cycling consisted of an initial 5-min denaturation step at 94°C, followed by 35 cycles of 1-min denaturation at 95°C, 1-min annealing at 55°C, and 1-min extension at 73°C. The cycling was followed by an extension step at 72°C for 10 min. Finally, 10 μl of PCR products were electrophoresed in 1% agarose gel stained with CYBR safe. DNA bands were visualized under UV light [6].
Culture of urogenital mycoplasmas/ureaplasmas
Vaginal swabs were immediately inoculated into SP4 broth medium prepared according to the previously described formulation. Swabs were rigorously vortexed for 5-10 seconds then filtrated through 0.45 mm pore size single use syringe filters in order to get rid of debris and to reduce bacterial and fungal contaminations. To ensure cultivation of M. hominis, M. fermentans, M. genitalium and the two Ureaplasma species, the SP4 medium was supplemented with either 0.5% arginine, 0.5% glucose, or 0.5% urea, depending on nutritional requirements. Reference Mycoplasma species Mycoplasma hominis PG21 (ATCC 23114), Mycoplasma fermentans PG18 (ATCC 19989), Mycoplasma genitalium G37 (ATCC 33530), Ureaplasma urealyticum (ATCC 27618) and Ureaplasma parvum (ATCC 27813) were used to control the culture procedure. All broth cultures were incubated at 37°C and were daily examined for growth and pH changing of the medium. Mollicutes growth was confirmed on agar plates maintained at 37°C with 5% CO2, and regularly observed microscopically for the appearance of mycoplasma and ureaplasma colonies [7].
Statistical analysis
Statistical analyses using excel tools (Microsoft, USA) were performed to determine the prevalence of each mycoplasma/ ureaplasma apart, as well as the prevalence of co-infections with 2 or more bacteria among the studied population. The Chisquare test and the Odds Ratio (OR) with a 95% Confidence Interval (CI) were computed in order to evaluate the possible association between the occurrence of urogenital mollicute infections and the different pathologies and to estimate their role as risk factors. Fisher’s exact test was assessed when the Chisquare test was not valid. A P value<0.05 was considered statistically significant and an OR value>1 indicated that mollicute infections might be a risk factor for the diseases. These tests were performed using the Statistical Package for Social Science (SPSS) 20.0 software. CIs of Odds ratio were calculated using the Baptista-Pike method [8].
Molecular detection of human genital Mycoplasma/ Ureaplasma spp
Genomic DNAs of all the type strains included in this study were extracted and used as PCR positive controls. Specificity of all the primer pairs was tested and confirmed. No amplification was detected with human DNA and no cross reactivity between mollicute species was observed. The conditions for amplification of each gene were optimized using the type strain DNAs prior to beginning the PCR using the clinical specimens. All protective measures were rigorously applied to prevent crosscontamination.
For each species, two PCR amplifications using 16S rRNA gene and a specific gene were performed. PCR amplification targeting the 16S rRNA of U. urealytium (313 bp), U. parvum (678 bp), M. hominis (335 bp) and M. fermentans (611 bp) was positive in 11, 10, 11, and 32 samples, respectively (Figure 1). Confirmation of molecular identification was achieved by a second-round of PCR amplification using species-specific primers. Amplification of Ureaplasma spp. specific Multiple Banded Antigen (MBA) gene was positive in 21 specimens. Indeed, 11 bands of 448 bp and 10 bands of 403 bp were revealed corresponding to U. urealyticum and U. parvum, respectively. For M. hominis, a segment of 297 bp of vaa gene was successfully amplified in 11 specimens. The detection of M. hominis was further confirmed by amplification of 510 pb fragment of another specific gene p120’ (data not shown). The specific IS-like element of M. fermentans was abundantly amplified at the expected size (205 bp) in 32 specimens. No positive PCR was observed for M. genitalium neither with 16S rRNA nor with MgPa targeting primers [9].
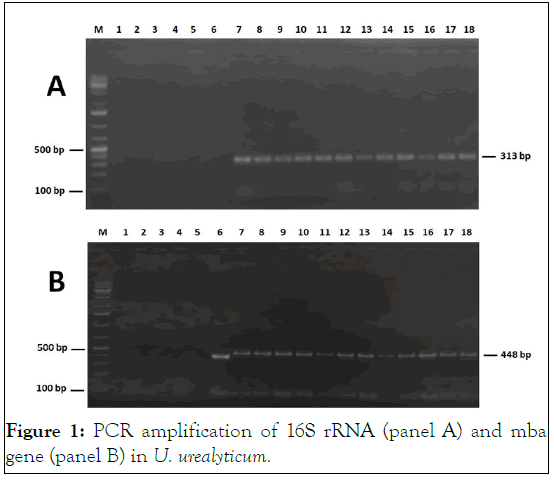
Figure 1: PCR amplification of 16S rRNA (panel A) and mba gene (panel B) in U. urealyticum.
All species combined, 64 positive PCR were detected in 50 patients among the 100 included in this study (50%). Single infections with M. fermentans, M. hominis, U. urealyticum and U. parvum were found to occur in 21% (21/100), 6% (6/100), 6% (6/100), and 4% (4/100) of the samples, respectively. Coinfection with two mycoplasma species was observed in 12% of the samples, and only one woman (1%) was found to be simultaneously infected with three mycoplasma species (Table 3) [10].
| Mollicute infections | Prevalence (%) |
|---|---|
| Single infection | 37 |
| M. hominis | 6 |
| M. fermentans | 21 |
| M. genitalium | 0 |
| U. urealyticum | 4 |
| U. parvum | 6 |
| Co-infection with two mollicute species | 12 |
| M. hominis+M. fermentans | 2 |
| M. hominis+U. ureaplasma | 1 |
| M. hominis+U. parvum | 1 |
| M. fermentans+U. urealyticum | 6 |
| M. fermentans+U. parvum | 2 |
| U. urealyticum+U. parvum | 0 |
| Co-infection with three mollicute species | 1 |
| M. fermentans+M. hominis+U. urealyticum | 1 |
| Total positive patients | 50 |
| Total negative patients | 50 |
| Total | 100 |
Table 3: Prevalence of mollicute infections according to PCR results.
Mycoplasma identification by culture
All type strains grew efficiently in broth and agar media. The SP4 liquid medium supplemented with arginine and inoculated with M. hominis was alkalinized, confirming the growth of this species through the hydrolysis of this amino acid. The growth of M. genitalium and M. fermentans was detected by the acidification of the SP4 liquid medium supplemented with glucose. Growth of ureaplasmas was detected by the change in colour of the SP4 medium supplemented with urea, indicating urea hydrolysis and ammonia release. The presence of characteristic colonies on SP4 agar plates further confirmed the growth of mycoplasma and ureaplasma reference strains (Figure 2).
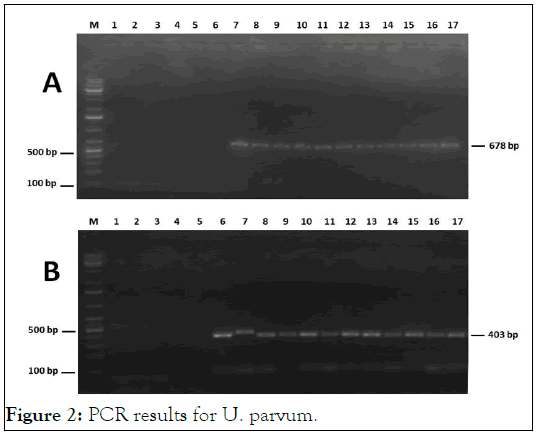
Figure 2: PCR results for U. parvum.
All clinical samples were treated as described above and subcultured in liquid and solid SP4 media for mycoplasmas and ureaplasmas isolation. By monitoring the pH change of the broth medium, 15 positive cultures were found among the 100 cultivated samples (15%). These cultures were diluted and then inoculated onto agar medium. Examination of the plates under a binocular magnifying glass revealed typical fried-egg colonies and brown urchin-like colonies which further confirmed mycoplasma and ureaplasma growth, respectively. We have been able to isolate some strains of M. hominis (N=11), M. fermentans (N=3) and Ureaplasma spp. (N=1). The isolated strains were cloned thrice and conserved at -80°C. No growth corresponding to M. genitalium could be demonstrated for any of the 100 samples (Figure 3).
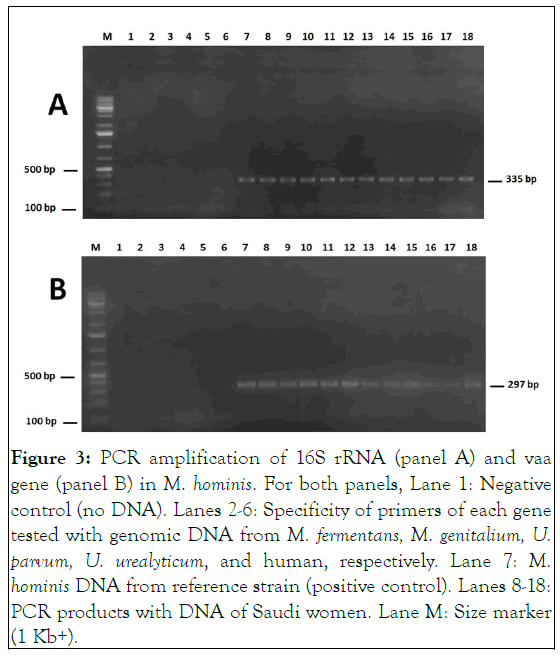
Figure 3: PCR amplification of 16S rRNA (panel A) and vaa gene (panel B) in M. hominis. For both panels, Lane 1: Negative control (no DNA). Lanes 2-6: Specificity of primers of each gene tested with genomic DNA from M. fermentans, M. genitalium, U. parvum, U. urealyticum, and human, respectively. Lane 7: M. hominis DNA from reference strain (positive control). Lanes 8-18: PCR products with DNA of Saudi women. Lane M: Size marker (1 Kb+).
With only 15 positive cultures versus 64 positive PCR amplifications, the bacteriological test is considered to be less sensitive than the molecular test for the detection of mollicute species. For M. fermentans and Ureplasma spp., PCR was found to be clearly more efficient than culture. However, for M. hominis, an identicalness between the two tests results was observed [11].
Association between mollicutes and urogenital troubles
M. fermentans, M. hominis, U. urealyticum and U. parvum detected in this study were all associated with patients presenting one or more urogynecological troubles. Therefore, no Mycoplasma was identified in an asymptomatic case (Figure 4).
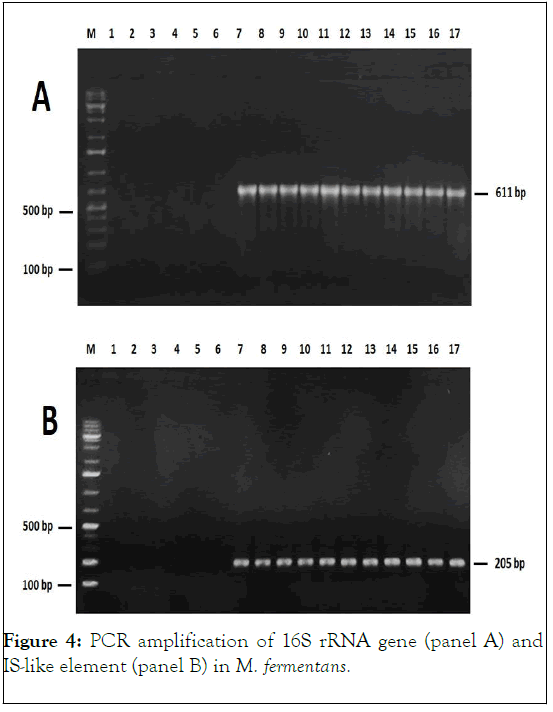
Figure 4: PCR amplification of 16S rRNA gene (panel A) and IS-like element (panel B) in M. fermentans.
Women suffering from pregnancy complications or genital troubles, were found to be frequently infected with mollicutes (70.45% and 71.87%%, respectively). Among the 20 women diagnosed with urological troubles, 12 (60%) were found to carry mollicutes. Among the detected mollicute species, M. fermentans was predominant among the three patient groups, pregnancy complications, genital disorders, and urogenital pathologies, with 14, 10, and 8 positive cases, respectively.
Regarding the six patients suffering from infertility (fourth group), only two of them were found to be infected, one with M. fermentans, and one with U. parvum. A diagram showing the distribution of mycoplasmas/ureaplasmas among the sick women is presented in Figure 5 [12].
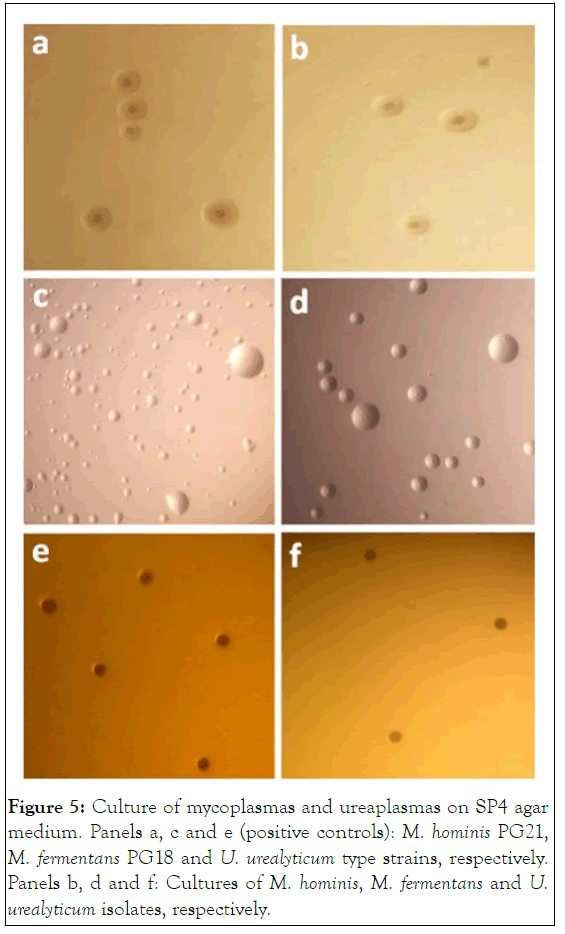
Figure 5: Culture of mycoplasmas and ureaplasmas on SP4 agar medium. Panels a, c and e (positive controls): M. hominis PG21, M. fermentans PG18 and U. urealyticum type strains, respectively. Panels b, d and f: Cultures of M. hominis, M. fermentans and U. urealyticum isolates, respectively.
A statistically significant association was found between U. urealyticum infections and pregnancy complications (P<0.05), and also between U. parvum infections and genital troubles (P<0.05). However, our study could not be conclusive with regard to any association between urogenital mollicute infections and infertility, given the limited number of sterile women (N=6). According to OR calculation for each mollicute species infection in the context of each pathology apart, we have found that a relative risk of pregnancy complications is related to infections with M. hominis (OR=1.611) and especially with U. urealyticum given the high OR value of 3.926. As for genital troubles, an occurrence risk was found to be correlated to infections with M. hominis (OR=1.914) and U. parvum (OR=3.692). This univariate analysis has also shown patients presenting M. fermentans infections to be more exposed to urological troubles (OR=1.556) (Table S3) [13].
M. genitalium, M. hominis, M. fermentans, U. urealyticum and U. parvum are bacterial species frequently isolated from the human urogenitourinary tract. With several other species, they are forming the urogenital mycoplasmas group. Some of them are considered commensals, however many others are found to be usually associated with serious localized infections in the urological and reproductive systems in both men and women. Urogenital mycoplasmas can be harmful enough to cause infertility and infant mortality.
In this paper, we searched for mollicute infection in a cohort of one hundred Saudi female patients showing different symptoms of urogenital troubles, complicated pregnancy, and infertility. Indeed, there is a paucity of data in Saudi Arabia regarding urogenital mycoplasma infections. Hence, this study was carried out in order to investigate the prevalence of urogenital mollicute infections and to determine if there is a meaningful correlation between these infections and the observed urogenital pathologies (Figure 6).
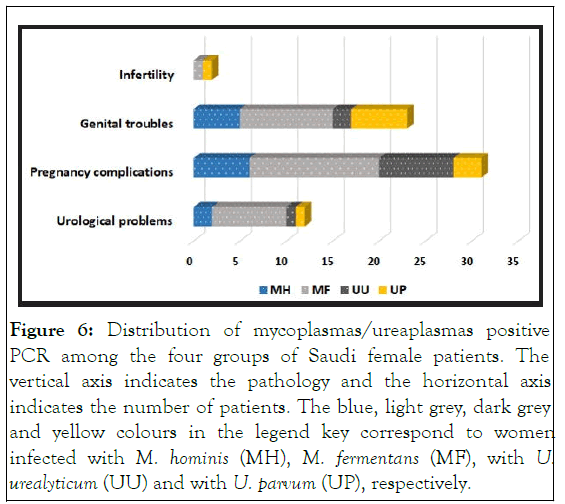
Figure 6: Distribution of mycoplasmas/ureaplasmas positive PCR among the four groups of Saudi female patients. The vertical axis indicates the pathology and the horizontal axis indicates the number of patients. The blue, light grey, dark grey and yellow colours in the legend key correspond to women infected with M. hominis (MH), M. fermentans (MF), with U. urealyticum (UU) and with U. parvum (UP), respectively.
Our findings clearly showed that PCR amplification is an efficient tool for the detection and species identification of urogenital mycoplasmas directly from vaginal swabs. PCR proved much more sensitive than culture (50% vs 15% detection rate), a finding reflecting the fastidious nature of these microorganisms, the complex medium, and the stringent conditions needed for their growth [14]. As a matter of fact, in a similar study previously conducted in Saudi Arabia, only two genital mycoplasmas were detected out of 263 specimens by culture method (<1%). The specificity of the primers used in our study was confirmed, since no cross reaction was observed between mycoplasma species. All the positive PCR targeting 16S rRNA of mollicute species were confirmed by a second-round PCR using species-specific genes. Positive amplification of mba gene served not only to detect ureaplasmas but also to distinguish the two biovars, U. urealyticum and U. parvum, based on amplicon size. The amplification of a segment of vaa gene and a sequence within the IS-like element allowed the selective detection of M. hominis and M. fermentans, respectively. Compared to previously reported methods using genus-specific primers our two-round PCR-based approach, though more timeconsuming, offers much more specificity. Moreover, lysis treatment of specimens proved to be a simple and effective alternative to DNA extraction. If a molecular diagnosis of urogenital mollicutes is required, such a method can greatly help to make the PCR procedure faster and easier.
Urogenital mollicute infections among Saudi female patients have previously been shown to occur frequently a finding which prompted the Saudi health authorities to start developing and setting up prevention and therapeutic strategies. It has been previously reported that urogenital mollicute infections have devastating effects on reproductive organs which could lead to infertility. The implication of mollicutes in female urogenital infections and their complications have been already demonstrated. Indeed, among 20 women presenting symptoms of urological troubles such as urethritis and dysuria, 12 were found to harbor mycoplasmas (M. fermentans and M. hominis) or ureaplasmas (U. urealyticum and U. parvum). Similarly, M. hominis and U. urealyticum were also detected in women with chronic urinary symptoms in a study previously conducted in Greece. Strikingly, and to our knowledge, our study is the first that detected such a high rate of M. fermentans infection in patients suffering from urogenital problems. Yet, the pathogenic role of M. fermentans is still controversial since this species has been sometimes associated with several diseases and sometimes considered as normal flora. According to our statistical analysis, M. fermentans infection was found to probably promote the occurrence of urological troubles. Such a singular result, which might represent a Saudi-specific issue, warrants to be further confirmed. In this respect, one should not neglect the possible implication of this species in other diseases such as rheumatic diseases AIDS and respiratory troubles.
With OR values approaching 4, U. urealyticum and U. parvum infections seem to constitute a risk factor of genital/ gynecological troubles. According to our results, a possible association could exist between Ureaplasma spp. and these diseases. Such a possibility was proposed and argued before in many studies around the world. In a Czech study including 225 women diagnosed with preterm pre-labour rupture of membranes, 68% were found to be colonized by U. urealyticum. Another Polish study reported a prevalence of 34% of U. parvum and U. urealyticum amongst 143 women with cervical pathology. Regarding M. hominis, statistics from Brazil have revealed no obvious causative relationship between infection with this species and the pathologies listed in this paper. However, M. hominis isolates recovered from Tunisian patients were shown to be associated with gynecological troubles and infertility. In fact, it has been proven that some strains of M. hominis were associated to infertility, while others are associated to gynecological infections.
The high rates of colonization with mycoplasmas/ureaplasmas observed in the studied population could be explained by their negligence in systematic diagnosis. This may explain the lack of appropriate antibiotic treatments administrated to the patients studied here. Indeed, only 7 women were treated with antibiotics. Of these, 5 were treated by antibiotics belonging to the beta-lactam class, to which mycoplasmas/ureaplasmas are naturally resistant because of the cell wall absence. This is in accordance with the positive PCR in most of these women which indicates the survival of these bacteria despite the prescription of antibiotics. The female patients included in this study should be urgently placed on antibiotic therapy using the appropriate antimicrobials such as tetracyclines, macrolides, and fluoroquinolones [14].
To sum up, this study revealed that urogenital mycoplasmas/ ureaplasmas are prevalent in the female patients suffering from urogenital troubles in Aseer region, Saudi Arabia. The tworound PCR amplification approach used herein proved to be a convenient method for the rapid detection and specific identification of urogenital mollicute infections. In doing so, Ureaplasma spp, were found to be significantly associated with genital/gynecological troubles. Strikingly, our study uncovered an unexpected high-rate of M. fermentans infection among Saudi women with various urogynecological conditions. The data garnered from this study will help the local health authorities applying the best diagnostic techniques, to correctly formulate treatment guidelines, and to establish a relevant and efficient control strategy.
The authors declare that there are no conflicts of interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yacoub E, Abusham AA, Abdul-Wahab OMS, Mardassi BBA, Boujemaa S, Khadraoui N, et al. (2023) Prevalence of Urogenital Mollicute Infections in Female Patients Originating from Aseer Region, Saudi Arabia. J Women’s Health Care. 12:641.
Received: 11-Sep-2020, Manuscript No. JWH-20-6453; Editor assigned: 17-Sep-2020, Pre QC No. JWH-20-6453(PQ); Reviewed: 01-Oct-2020, QC No. JWH-20-6453; Revised: 24-Apr-2023, Manuscript No. JWH-20-6453(R); Published: 22-May-2023 , DOI: 10.35248/2167-0420.23.12.641
Copyright: © 2023 Yacoub E, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.