Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2023)Volume 13, Issue 5
Diabetes mellitus is a progressive chronic disease associated with enhanced levels of fasting glucose, HbA1C, and insulin resistance. Despite valuable recommendations of changes in diet and lifestyle, the risk of developing diabetes type II continues to increase. Risk factors such as elevated levels of oxidative stress and chronic inflammation, intestinal dysbiosis, omega-3-fatty acids dysfunction, and reduced secretion of insulin contribute to the development and progression of diabetes. Addressing one of these defects at a time may not produce an optimal benefit. To prevent and improved treatment of diabetes, this review proposes
(a) changes in diet and lifestyle,
(b) supplementation with a micronutrient mixture which would simultaneously reduce oxidative stress and chronic inflammation,
(c) probiotics with prebiotics which would reverse the intestinal dysbiosis,
(d) omega-3-fatty acids which would directly activate the insulin receptor-linked signaling protein AKT leading to the entry of glucose inside the cells in patients with insulin resistance, and
(e) collagen peptides which increase the secretion of insulin by inhibiting Dipeptidyl Peptidase-4 (DPP-4) activity.
Despite diabetic medications, complications such as retinopathy, nephropathy, peripheral neuropathy, and heart disease continue to develop due to resistance to medications. The proposed plan in combination with drugs may improve their effectiveness.
Diabetes; Oxidative stress; Chronic inflammation; Intestinal dysbiosis; Omega-3; Glycemic index
Diabetes mellitus is a progressive chronic disease which exhibits higher levels of fasting glucose, HbA1C, and insulin resistance than normal levels. Despite recommendations of diet and lifestyle, the incidence of diabetes type II continues to increase in the USA and worldwide. In 2021, The Center for Disease Prevention and Control (CDC) reported that 19 million Americans had diabetes in 2010; this number enhanced to 37.3 million in 2019. In 2018, 88 million Americans had prediabetes, this number increased to 96 million in 2019. In 2022, WHO (World Health Organization) reported that the number of diabetes cases in the world rose from 108 million in 1980 to 463 million in 2019. Human attitudes with respect to diet and lifestyle are not easy to alter, which could account for the lack of impact on the incidence of diabetes. In 2019, 1.6 million Americans aged 20 years and younger had type I diabetes. Type II diabetes is the 5th leading cause of death globally. The prevalence of diabetes type II in the USA depends upon the ethnicity. In 2019, it was 14.8% among Indian/Alaska Natives, 12.1% among blacks, 11.8% among Hispanic, 9.5% among Asian, and 7.5% among Whites [1]. To decrease the risk of diabetes type II, it is necessary to develop and implement an effective and easily adaptable guideline. To achieve this, key early cellular events that initiate the development of diabetes should be identified. Elevated oxidative stress and chronic inflammation are early cellular events that lead to the development and progression of this disease and its associated complications [2-25]. In addition, intestinal dysbiosis (increase in the number of pathogenic bacteria and decline in the number of beneficial bacteria) participates in the development and progression of diabetes [26-30]. Increased activity of DPP-4 (Dipeptidyl Peptidase-4) inhibited 2 gut-generated incretin hormones which facilitate the secretion of insulin from the pancreas to the blood in patients with diabetes type II [31]. Insulin resistance is one of the major contributors to the development of diabetes type II, and it also develops after prolonged treatment of diabetes type I. Thus, multiple independent cellular defects initiate and promote diabetes and its complications. Therefore, resolving a single cellular defect may not be sufficient to have an optimal beneficial effect in the prevention or treatment of diabetes.
Although inhibitors of DPP-4 such as sitagliptin and vildagliptin approved by the FDA are commonly used for improving glycemic index, they produce some side effects. Therefore, a non-toxic inhibitor of DPP-4 would be useful in diabetes.
Despite oral drugs and insulin therapy, diabetes continues to progress slowly, and diabetic complications such as retinopathy, nephropathy, peripheral neuropathy, and heart disease develop in many cases. In 2016, 130,000 patients had lower extremities amputated, 438,000 had ischemic heart disease, and 313,000 had stroke [1]. The diabetic medications have improved glycemic index, but after prolong treatment, the patients become resistance to medications. Since these drugs do not influence the levels of oxidative stress and chronic inflammation, the disease continues to progress leading to diabeticrelated complications.
This review briefly describes the role of oxidative stress, chronic inflammation, intestinal dysbiosis, omega-3-fatty acid dysfunction, enhanced activity of DPP-4, and insulin resistance in the development and progression of diabetes type II. This review suggests that the combination of a mixture of micronutrients, probiotics with prebiotics, omega-3-fatty acids, and collagen peptides, may decrease the risk of development and progression of diabetes and its associated complications. Application of such a multiple nutritional approach together with diabetic medications may prolong the effectiveness of medications.
Role of oxidative stress and chronic inflammation in type II and type I diabetes
Role of oxidative stress in the development of diabetes type II: Several investigations have revealed that markers of oxidative stress such as DNA adduct 8-hydroxy-2’-deoxyguanosine (8-OHdG), lipid peroxidation product Thiobarbituric Acid-Reactive Substances (TBARS), protein oxidation products nitrotyrosine and carbonyl increased and the levels of antioxidant enzymes decrease in patients with hyperglycemia [12]. An elevated production of Reactive Oxygen Species (ROS) was also confirmed in the culture of beta cells of the pancreas [32]. ROS caused reduction in the expression of insulin genes leading to a decreased production of insulin [33]. In addition, prolonged exposure to ROS could reduce the number of beta cells of the pancreas. Enhanced oxidative stress also leads to hyperglycemiainduced insulin resistance by damaging insulin receptors or itsmediated signaling AKT which prevents the translocation of Glucose Transporter-4 (GLUT-4) to the cell surface membrane, and thereby, inhibiting the entry of glucose uptake inside the cells for generating energy. Hyperglycemia-induced elevated oxidative stress is due to a non-enzymatic glycosylation which leads to the formation of Advanced Glycosylation End-Products (AGEs) [34]. Enhanced accumulation of AGEs contributes to vascular damage leading to diabetic-related complications such as retinopathy, nephropathy, and atherosclerosis [35]. Mitochondrial dysfunctions, which includes a defect in biogenesis, number, morphology, and process of fusion and fission, occurs in individuals who develop insulin insufficiency, insulin resistance, and who are obese [36,37]. Hyperglycemia generates excessive amounts of ROS, which can impair oxidative phosphorylation of mitochondria in muscle cells causing insulin resistance [28,37]. Beta cells of the pancreas exposed to chronic hyperglycemia decrease the synthesis of insulin in an animal models of diabetes type II [33]. A study has shown that increased oxidative stress leads to the development of chronic hyperglycemia-induced insulin resistance [34]. This was further confirmed in an experiment in which incubation of primary adipocyte cells in culture with a high concentration of glucose produced enhanced oxidative stress [38]. In 3T3-Li adipocyte cells in culture, oxidative stress-induced insulin resistance occurs by inhibiting the translocation of GLUT- 4 from the cytoplasm to the plasma membrane of the cell [39]. A similar observation was made in the intact rat muscle [40]. Several investigations and reviews also have confirmed the role of enhanced oxidative stress in in the initiation and progression of diabetes [2- 11]. The fact that elevated levels of markers of oxidative damage were observed in prediabetic patients may further support the role of enhanced oxidative stress in diabetes type II [5]. Oxidative damage of cells, if not fully healed, leads to chronic inflammation which releases free radicals, pro-inflammatory cytokines, complement proteins, adhesion molecules, and prostaglandins all of which are toxic to cells.
Role of oxidative stress in the progression of diabetes type I: Hyperglycemia can cause an increased levels of oxidative stress which enhances the risk of developing microvascular and macrovascular complications in diabetes type I. In Non-Obese Diabetic (NOD) mouse models, oxidative stress-induced damage is more pronounced in the beta-cells of the pancreas and vascular tissue compared to diabetic-resistance NOD mice [41]. The levels of markers of oxidative stress such as Malondialdehyde (MDA) and protein carbonyl were progressively higher in the plasma of diabetic children and adolescents than in control subjects. The activity of glutathione peroxidase and the level of glutathione were also progressively declined in the erythrocytes of diabetic children and adolescents. Furthermore, the levels of vitamin E and beta-carotene were low in the plasma of diabetic children. These results show that enhanced oxidative stress also participates in the pathogenesis of type I diabetes [42]. The levels of markers of oxidative damage were increased in parents of children as well as children with diabetes type I [43-45].
Role of chronic inflammation in the development of diabetes type II: Chronic inflammation also participates in the pathogenesis of type II diabetes [46]. The level of C-Reactive Protein (CRP) in plasma increases in type II diabetes with large size Low Density Lipoprotein (LDL) cholesterol, (LDLc A), or small size LDLc (LDLc B) (small) compared to control subjects. The levels of the proinflammatory cytokine IL-6 enhances in diabetes type II with large size LDLc [47]. The levels of CRP and proinflammatory cytokines IL-6, and TNF-alpha were enhanced in the plasma of patients with metabolic syndrome and diabetes type II [48,49]. In addition, the levels of tumor necrosis factor-alpha (TNF-alpha) and monocyte chemotactic protein 1 (MCP-1) were elevated in patients with diabetes type II [50- 52]. Chronic hyperglycemia associated with diabetes type II can cause diabetic complications such as retinopathy, nephropathy, neuropathy, cardiovascular disease, and blood vessel damage [53,54]. Increased level of a pro-inflammatory cytokine TNF-alpha was associated with obesity as well as with insulin resistance and diabetes type II [55]. Other reviews and investigations have suggested that chronic inflammation also participates in the initiation and progression of diabetes [13-21].
Role of autoimmune and chronic inflammation in the development of diabetes type I
Diabetes type 1 is considered an autoimmune disease in which damage to beta cells of the pancreas occurs leading to the cessation of insulin. Both CD4+ and CD8+ T cells participate in the development of diabetes type I in an animal models [56]. Both humoral and cellular immunity play a role in the pathogenesis of diabetes type I [57, 58]. Activated macrophages release excessive amounts of proinflammatory cytokines such as IL-1beta and TNF-alpha which cause inflammatory damage to beta cells of the pancreas [58]. The combination of three inflammatory molecules INF-gamma, IL-6, and TNF-alpha causes damage to beta cells of the pancreas, upregulates the activity of inducible Nitric Oxide Synthase (iNOS) that produces increased levels of Nitric Oxide (NO) which together with ROS can cause death of beta cells of the pancreas [59,60]. These results show that attenuation of oxidative stress and chronic inflammation may be useful in reducing the progression of diabetes type I.
Role of oxidative stress and chronic inflammation in the development of diabetic-related complications
Retinopathy: Diabetic retinopathy occurs in poorly controlled diabetes. Increased oxidative stress and chronic inflammation participate in the development of diabetic retinopathy by damaging the microvessels. The levels of several inflammatory cytokines and chemokines were enhanced in the serum and ocular samples (vitreous and aqueous humor) from diabetic patients [61-63]. Reactive retinal gliosis produces proinflammatory cytokines which damage retinal cells. The level of Vascular Endothelial Growth Factor (VEGF) increases, which contributes to the development of vascular permeability and angiogenesis in severe diabetic retinopathy [64,65]. This eye disease occurs in 50% of diabetic patients 10 years after diagnosis, and in 90% after 25 years of diagnosis. Retinopathy is the primary cause of blindness [22,23].
Nephropathy: Diabetic Nephropathy (DN) occurs because of the damage to microvessels of the kidney by increased oxidative stress and chronic inflammation, causing persistent albuminuria and gradual decline in Glomerular Filtration Rate (GFR). DN is the leading cause of End-Stage Renal Disease (ESRD) which occurs approximately 20 years after the onset of diabetes and accounts for 45% of diabetic cases. The incidence of ESRD was similar in both diabetes type I and diabetes type II [29]. Both oxidative stress and the products of chronic inflammation such as pro-inflammatory cytokines, adhesion molecules, and chemokines play an important role in the development and progression of DN [24]. Thus, both elevated oxidative stress and chronic inflammation participate in the development and progression of DN and end-stage renal disease [66].
Peripheral neuropathy: Diabetic peripheral neuropathy is one of the vascular complications of diabetes. This peripheral nerve disease is very painful and disabling. Enhanced oxidative stress and chronic inflammation-mediated damage to neurons, glia cells, and microvessels lead to the development and progression of diabetic neuropathy [25]. Hyperglycemia-induced increased oxidative stress and advanced glycation products also play a major role in the development and progression of diabetic neuropathy [67].
Potential steps involved in the development of diabetes type II
Impairment of insulin receptors: Enhanced oxidative stress and chronic inflammation Damage the insulin receptor, which prevents the binding of insulin to its receptors and insulin receptor-mediated stimulation of AKT signaling pathways, and thereby, inhibits the translocation of Glucose Transporter protein-4 (GLUT-4) stored in the vesicles in the cytoplasm to the cell surface membrane [68,69]. This process prevents the uptake of glucose leading to accumulation of glucose in the blood causing hyperglycemia and eventually diabetes type II. Continued oxidative stress and chronic inflammation also damage ability of the pancreas to produce insulin [12]. These investigations reveal that the simultaneous reduction of oxidative stress and chronic inflammation may be useful for the attenuation of the development and progression of diabetes.
Intestinal dysbiosis: Even though Dr. Hippocrates, a physician and philosopher stated, “All diseases begin in the gut” some 2500 years ago, the importance of intestinal microorganisms in human health and disease was demonstrated much later. The intestinal dysbiosis occurs due to decline in the number of beneficial bacteria and overgrowth of pathogenic bacteria. The growth of harmful bacteria generates pro-inflammatory cytokines which are toxic to the cells, and deprive the body from certain B-vitamins, vitamin K, neurotransmitters, and short-chain fatty acids which are essential for maintaining good health. Intestinal dysbiosis is also associated with diabetes type II and type I [26]. It also participates in the rapid progression of insulin resistance in diabetes type II [70]. The intestinal dysbiosis also decreases the production of short-chain fatty acids such as butyric acid, propionic acid, and acetic acid [71]. Butyric acid has diverse biological functions which include improving intestinal barrier integrity, pancreatic beta cell proliferation, and insulin sensitivity, and reducing glycemia and body weight [72]. Intestinal dysbiosis can also increase the production of other metabolites such as branched amino acids which causes insulin resistance that can lead to the development of diabetes type II [73,74]. It also causes inflammation and enhances intestinal permeability. Intestinal dysbiosis has been observed in animal models and in patients with diabetes type II and its complications such as retinopathy, nephropathy, peripheral neuropathy, cardiovascular diseases, and coronary artery disease [75]. Severity of intestinal dysbiosis is related to the severity of diabetes type II. Therefore, restoring the number of beneficial bacteria and reducing the number of pathogenic bacteria may be needed to decrease the risk of development and the rate of progression of diabetes.
Omega-3 dysfunction: Increased oxidative stress may oxidize omega- 3-fatty acids which become ineffective or even can produce harmful effects [76]. In a randomized, placebo controlled, double blind clinical trial involving 92 patients with diabetes type II, the effect of omega-3 on glycemic index was investigated. The results showed that daily supplementation with 2,714 mg for a period of two-months reduced HbA1c without affecting the activity of antioxidant enzymes [77]. Another randomized placebo controlled trial involving 51 subjects aged 10-18 years with diabetes type I revealed that supplementation with 600 mg omega-3 (180 mg EPA and 120 mg DHA) for 12 weeks increased the level of Flow Mediated Dilation (FMD) and reduced the level of triglycerides, and thereby, may reduce the risk of cardiovascular diseases in these patients [78].
DHA is more effective than EPA in reducing markers of chronic inflammation [79]. Omega-3 acts as an endocannabinoid ligand such as anandamide and 2-AG which activates endocannabinoid receptors CB1 and CB2 [80]. DHA/EPA promotes translocation of GLUT- 4 from cytoplasmic vesicles to the plasma membrane by causing phosphorylation of insulin receptor-linked serine/thereonine protein kinase (AKT) in both normal and insulin resistance individuals [81]. Translocated GLUT-4 allows the entry of glucose in adipocytes which use glucose for generating energy.
Reduced secretion of insulin from the pancreas: Two gut-generated incretin hormones, which facilitate the secretion of insulin from the pancreas, are inhibited by the rise in DPP-4 (Dipeptidyl Peptidase-4) activity in patients with diabetes type II [31]. Therefore, a nontoxic inhibitor of Protein-4 would be helpful in the prevention and improved management of diabetes.
How to simultaneously reduce oxidative stress and chronic inflammation in diabetes
As discussed in sections 2-4, increased oxidative stress and chronic inflammation play an important role in the development and progression of diabetes and its complications. Therefore, the role of antioxidants, which reduce oxidative stress and chronic inflammation, in the prevention and progression of diabetes was evaluated. Most clinical studies have been performed to evaluate the role of individual antioxidants for a short period of time in improving glycemic index. Such studies have yielded an inconsistent beneficial effects in patients with diabetes. Some examples are described here.
Administration of antioxidants: This section has focused on the effects of individual or more than one antioxidant in the prevention and progression of diabetes in humans.
Vitamin A: A review has suggested that supplementation with vitamin A may be helpful in patients with diabetes type II who suffer from vitamin A deficiency [82]. Treatment with all-trans-retinoic acid increased insulin sensitivity [83].
Vitamin C: Administration of high doses of vitamin C did not improve endothelial dysfunction and insulin resistance [84]. Analysis of 26 observational studies and 12 randomized controlled trials revealed that supplementation with vitamin C reduced fasting glucose levels but failed to decrease the levels of HbA1c [85].
Coenzyme Q10: A review of seven clinical trials showed that supplementation with coenzyme Q10 had no beneficial effects on glycemic control, lipid profiles or blood pressure in patients with diabetes [86].
Alpha-lipoic acid: Supplementation with alpha-lipoic acid alone reduced polyneuropathy associated with diabetes [87].
Resveratrol: Analysis of several clinical studies on supplementation with resveratrol for a period of 4-5 weeks had no impact on the levels of fasting glucose and HbA1c in patients with diabetes type II. No adverse effects were reported [88].
Curcumin: Analysis of clinical investigations revealed that supplementation with curcumin for a period of 7 weeks-10 weeks decreased the levels of lipid peroxidation, fasting glucose, HbA1c, triglycerides, total cholesterol, LDLc and C-reactive protein, systolic blood pressure, and increased the levels of HDLc [89].
Quercetin: Supplementation with quercetin had no effect on the levels of fasting glucose, HbA1c, serum insulin, and lipid profile. Another clinical study showed that quercetin treatment improved glycemic control. Quercetin activates Adenosine Monophosphate Kinase (AMPK) in skeletal muscles which stimulates AKT that causes translocation of GLUT-4 from the cytoplasm to the cell membrane which then allows the entry of glucose inside the cells [90,91]. Most clinical studies on the effects of individual antioxidants in diabetes have been performed for a period of 4 weeks-12 weeks.
Despite overwhelming evidence, which supports the role of oxidative stress in experimental models of diabetes as well as in human diabetes, large well-designed long-term clinical trials with antioxidants such as vitamin E and alpha-lipoic acid individually failed to yield benefits in the management of diabetes [92]. A review of clinical studies on the effect of vitamin E, vitamin C, coenzyme Q10, alpha-lipoic acid, and L-carnitine concluded that these antioxidants individually produced no significant benefits in the management of diabetic complications [93]. In another clinical study, treatment with vitamin E or vitamin C in combination with metformin for a period of 90 days improved glycemic control [94].
A few potential reasons for the failure of a single antioxidant to yield consistent beneficial effects in patients with diabetes are described here.
• Diabetic patients have an elevated level of oxidative environment. Supplemented single antioxidant in such an environment would be oxidized which then would act as a pro-oxidant rather than as an antioxidant.
• Different antioxidants are distributed differently and in different amounts in the subcellular compartments of the cells. Supplemented single antioxidant cannot accumulate in all subcellular parts of the cell in sufficient amounts to provide an adequate protection against oxidative damage.
• Alpha-tocopherol is a more efficient scavenger of free radicals in reduced oxygen pressure, whereas beta-carotene and vitamin A are more effective in higher oxygen pressure [95]. The pressure of oxygen varies from one organ to another and within the cells in the same organ [96]. A single antioxidant cannot protect against oxidative damage under such a varying oxygen pressure in the body.
• Elevation of both antioxidant enzymes and dietary and endogenous antioxidant compounds is essential to attenuate simultaneously oxidative stress and chronic inflammation because they act by different mechanisms. Antioxidant compounds neutralize free radicals by donating electrons to those molecules with unpaired electrons, whereas antioxidant enzymes remove hydrogen peroxide (H2O2) by catalysis, converting them to water and oxygen. Supplementation with a single antioxidant alone cannot achieve this goal.
• Supplementation with a single antioxidant cannot protect molecules against oxidative damage in both the aqueous and lipid environment of the cells.
Proposed micronutrient mixture elevates the levels of antioxidant enzymes and antioxidant compounds
We have proposed that the levels of antioxidant enzymes and dietary and endogenous antioxidant compounds should be simultaneously elevated to decrease oxidative stress and inflammation at the same time [97]. Oral administration of the proposed mixture of micronutrients may enhance the levels of antioxidant compounds; however, elevating the levels of antioxidant enzymes requires an activation of Nrf2. The process of activation of Nrf2 and its role in enhancing antioxidant enzymes is briefly described here.
ROS-induced activation of Nrf2: Under normal physiological conditions, ROS (Reactive Oxygen Species) is essential to activate Nrf2. Activated Nrf2 dissociates itself from Keap1-CuI-Rbx1 complex in the cytoplasm and then migrates to the nucleus where it heterodimerizes with a small Maf protein and binds with ARE (Antioxidant Response Element) leading to increased transcription of genes coding for several enzymes including antioxidant enzymes such as glutathione peroxidase, catalase, and superoxide dismutase [98-100].
Presence of ROS-resistant Nrf2 and its activation: Nrf2 becomes resistant to ROS during chronic oxidative stress [101-103]. Elevated oxidative stress is observed in diabetic patients despite the availability of Nrf2, suggesting that ROS has failed to activate Nrf2 in diabetes. The importance of Nrf2 activation was demonstrated in experiments in which the rate of wound healing in streptozotocin-induced diabetic mice lacking Nrf2 (-/-) was slowed down compared to diabetic mice with Nrf2 (+/+). Activation of Nrf2 by pharmacological agents improved the rate of diabetic wound healing [104]. Some antioxidant compounds, such as vitamin E and genistein, alpha-lipoic acid, curcumin resveratrol omega-3-fatty acids, glutathione, NAC, and coenzyme Q10 activate ROS-resistant Nrf2 [105-114]. Activation of Nrf2 [115,116] and some antioxidant compounds also decreased chronic inflammation [117-124].
The mechanisms of activation of ROS-resistance Nrf2 by certain antioxidants are not known. A study suggests that different antioxidants activate Nrf2 by altering different microRNAs [125]. For example, antioxidants such as C66, an analog of curcumin, can activate Nrf2 by upregulating miR-200a that inhibits its target protein Keap1resulting in the increased levels of Nrf2 available for activation, the same antioxidant also can activate Nrf2 by downregulating miR- 21 which increases the transcription of Nrf2 which then become available for activation [126].
Proposed plan to prevent the development and progression of diabetes type II
Diet and lifestyle changes: Dietary recommendations include daily consumption of a low fat and high fiber diet with plenty of fruits and vegetables and reduction of sugar intake. Decrease the consumption of very rich protein diet because it enhances the production of branched amino acids (leucine, isoleucine, and valine) which may increase the risk of diabetes type II [127].
Lifestyle recommendations include daily moderate exercise such as walking for 30 minutes 5 days a week, stopping cigarette smoking and vaping (e-cigarette), reducing stress by yoga or vacation, and limiting intake of caffeine because high doses of caffeine may interfere with the repair of DNA damage. Avoid exposure to air pollution containing tiny particles, because it increases the risk of diabetes type II by elevating oxidative stress and chronic inflammation [128,129].
Reduction of oxidative stress and chronic inflammation by a micronutrient mixture: Failure of individual antioxidants to yield consistent beneficial effects in diabetic patients led us to suggest a micronutrient mixture containing vitamin A, natural mixed carotenoids, vitamin C, vitamin E, curcumin, resveratrol, alpha-lipoic acid, coenzyme Q10, synthetic antioxidant N-Acetylcysteine (NAC), curcumin, resveratrol, quercetin, green tea extract, vitamin D3, all B-vitamins, and minerals selenium and zinc for prevention and improved treatment of diabetes. This micronutrient mixture would simultaneously attenuate oxidative stress and chronic inflammation by enhancing the levels of antioxidant enzymes through activation of the Nrf2 pathway as well as the levels of dietary and endogenous antioxidant compounds [130] (Figure 1).

Figure 1: Diagrammatic representation to show that the proposed micronutrient mixture simultaneously reduces oxidative stress and chronic inflammation and leads to a reduced risk of developing diabetes and progression of diabetic complications ARE: Antioxidant response element, Nrf2: Nuclear factor-erythroid-2-related factor-2
ARE: Antioxidant response element, Nrf2: Nuclear factor-erythroid- 2-related factor-2
Reversal of intestinal dysbiosis by probiotics with prebiotics: Administration of probiotics with prebiotics may reverse intestinal dysbiosis by enhancing the number of beneficial bacteria and reducing the number of pathogenic bacteria, and thereby, decrease the risk of development and progression of diabetes [131-133]. Addition of prebiotics to probiotics was considered essential because they provide substrate to bacteria for fermentation which is necessary to produce short-chain fatty acids such as butyric acid (Figure 2).
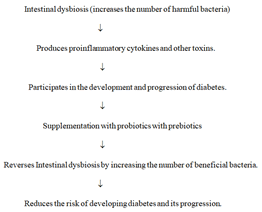
Figure 2: Diagrammatic representation to show intestinal dysbiosis increased the risk of developing diabetes and its rate of progression, and supplementation with probiotics with prebiotics reverses the intestinal diabetes by increasing the number of beneficial bacteria
Omega-3 fatty acids improve glucose metabolism in patients with insulin resistance diabetes: In patients with diabetes type II and type I, insulin resistance develops due to damage to the insulin receptor or its linked cell signaling molecule AKT by increased oxidative stress. If the insulin resistance issue is not resolved, it can lead to diabetic-related complications such as nephropathy, retinopathy, and peripheral neuropathy. Supplementation with omega-3 fatty acids can improve glucose metabolism in insulin resistant patients. Omega 3 promotes translocation of GLUT-4 from cytoplasmic vesicles to the plasma membrane by causing phosphorylation of insulin receptorlinked serine/thereonine protein kinase (AKT) in insulin resistance patients with diabetes [81]. Translocated GLUT-4 allows the entry of glucose in the cells which use glucose for generating energy (Figure 3).
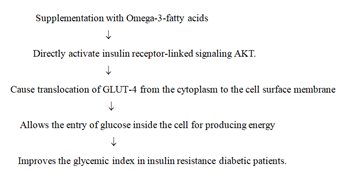
Figure 3: Diagrammatic representation to show that omega-3-fatty acids directly activate the insulin receptor-linked signaling molecule AKT, causing translocation of GLUT-4 from the cytoplasm to the cell surface membrane, which then allows the entry of glucose into the cell for generating energy in patients with insulin resistance diabetes
Collagen peptides inhibit DPP-4 activity: Increased activity of Dipeptidyl Peptidase-4 (DPP-4) degrades the two gut-generated incretin hormones, Glucagon-Like Peptide-1 (GLP-1) and Glucose- Dependent Insulinotropic Polypeptide (GIP), causing a decline in the secretion of insulin from the pancreas to the blood in patients with diabetes type II [31]. Collagen peptides increase the secretion of insulin by inhibiting the enzyme DPP-4 without toxicity [134] (Figure 4).
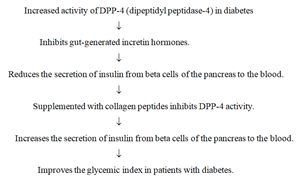
Figure 4: Diagrammatic representation to show that collagen peptides increase insulin secretion from the pancreas to the blood by inhibiting the enzyme DPP-4 (dipeptidyl peptidase-4) in patients with diabetes
Proposed suggestions for improving the efficacy of diabetic medications in the treatment of diabetes
Treatment with oral diabetic drugs: All diabetic medications reduce the levels of fasting blood glucose, HbA1c, and enhance insulin sensitivity in patients with diabetes. There are different classes of oral drugs which maintain glycemic control by different mechanisms [135].
They include alpha-glucosidase inhibitors (break down of carbohydrate and sugar in the intestine), biguanides-metformin (increases insulin sensitivity and decreases the production and release of glucose by the liver), Dipeptidyl Peptidase-4 (DPP-4) inhibitors (enhance secretion of insulin from the pancreas to the blood), agonists of GLP- 1 (Glucagon-Like Peptide-1) receptor (stimulate the beta-cell of the pancreas to produce and secrete more insulin), dopamine receptor-2 agonists (decrease insulin resistance and the production of glucose by the liver), meglitinides (stimulate the pancreas to produce more insulin), Sodium-Glucose Transport Protein-2 (SGLT2) inhibitors (block the re-absorption of glucose by the kidney), sulfonylureas (stimulate insulin production and release from the beta-cells of the pancreas), Thiazolidinediones (TZDs) (increase insulin sensitivity, decrease the production of glucose by the liver, accumulation of free fatty acids, inflammatory cytokines, and preserve the function of beta cells of the pancreas). Generally, insulin is administered to patients with diabetes II who become resistance to all oral drugs to improve glycemic index. Although such treatment is effective in attenuating the levels of fasting glucose and HbA1c, the risk of hypoglycemia enhances [136]. When patients with diabetes type I become resistance to insulin, diabetic oral drugs such as metformin, SGLT-2 inhibitors, and agonists of GLP-1 receptor are added to maintain a good glycemic index [137-139].
Collagen peptides in combination with oral diabetic medications: In a randomized placebo-controlled clinical trial, supplementation with collagen peptides in combination with oral diabetic drugs reduced the levels of fasting glucose and HbA1c, and increased insulin sensitivity more than those found in patients treated with oral drugs alone [140,141]. In patients with diabetes type I, the damaged pancreas cannot produce insulin; therefore, treatment with collagen peptides, which increases the secretion of insulin from the pancreas to the blood by inhibiting the enzyme DPP-4, may be useful in this disease.
Proposed plans to improve the efficacy of diabetic medications: Despite the use of different classes of oral diabetic drugs and insulin, the disease continues to progress slowly, and cases of diabetic complications do occur. One of the reasons could be that these drugs do not affect the levels of oxidative stress and chronic inflammation, severity of intestinal dysbiosis, insulin resistance, and omega-3 dysfunction, The proposed plan suggests that the administration of combination of diabetic medications together with changes in diet and lifestyle, a micronutrient mixture, probiotics with prebiotics, omega-3 fatty acids, and collagen peptides may be more effective in improving treatment of diabetes type II and type I than the diabetic medications alone (Figure 5).
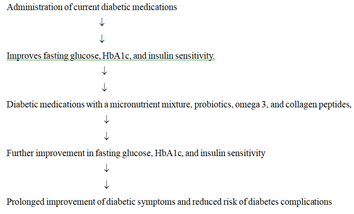
Figure 5: Diagrammatic representation for improving the efficacy of diabetic medications by a combination of changes in diet and lifestyle, a micronutrient mixture, probiotics with prebiotics, omega-3 fatty acids and collagen peptides in patients with diabetes type II and type I
Diabetes mellitus is a progressive chronic disease which is associated with enhanced levels of fasting glucose, HbA1C, and insulin resistance compared to normal subjects. Despite valuable dietary and lifestyle recommendations, the incidence of diabetes type II continues to increase in the USA and the world-wide. The proposed plan, which includes a micronutrient mixture, probiotics with prebiotics, omega-3-fatty acids, and collagen peptides may attenuate the risk of the development and progression of diabetes type II and the risk of developing diabetic-related complications. Despite the use of different classes of diabetic oral drugs and insulin, the disease progresses slowly, and in many cases diabetic complications such as retinopathy, nephropathy, peripheral neuropathy, and heart disease continue to develop. The proposed plan, which includes a combination of a mixture of micronutrients, probiotics with prebiotics, omega-3 fatty acids and collagen peptides together with oral drugs and/or insulin may be more effective in the management of diabetes type II and type I than diabetic medications alone.
Since it is a review manuscript, ethical statement is not needed. Any ethical statement related to a review paper has been met.
The author is Chief Scientific Officer of Engage Global of Utah. This company sells nutritional products to consumers.
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sector.
None.
Diabetes mellitus is a progressive chronic disease associated with enhanced levels of fasting glucose, HbA1C, and insulin resistance. Despite valuable recommendations of changes in diet and lifestyle, the risk of developing diabetes type II continues to increase. Risk factors such as elevated levels of oxidative stress and chronic inflammation, intestinal dysbiosis, omega-3-fatty acids dysfunction, and reduced secretion of insulin contribute to the development and progression of diabetes. Addressing one of these defects at a time may not produce an optimal benefit. To prevent and improved treatment of diabetes, this review proposes
(a) changes in diet and lifestyle,
(b) supplementation with a micronutrient mixture which would simultaneously reduce oxidative stress and chronic inflammation,
(c) probiotics with prebiotics which would reverse the intestinal dysbiosis,
(d) omega-3-fatty acids which would directly activate the insulin receptor-linked signaling protein AKT leading to the entry of glucose inside the cells in patients with insulin resistance, and
(e) collagen peptides which increase the secretion of insulin by inhibiting Dipeptidyl Peptidase-4 (DPP-4) activity.
Despite diabetic medications, complications such as retinopathy, nephropathy, peripheral neuropathy, and heart disease continue to develop due to resistance to medications. The proposed plan in combination with drugs may improve their effectiveness.
Citation: Kedar N Prasad (2023) Prevention and Improved Treatment of Diabetes using a Micronutrient Mixture, Probiotics, Omega3, and Collagen Peptides. J Nutr Food Sci. 13:49.
Received: 02-Oct-2023, Manuscript No. jnfs-23-28179; Editor assigned: 04-Oct-2023, Pre QC No. jnfs-23-28179 (PQ); Reviewed: 18-Oct-2023, QC No. jnfs-23-28179; Revised: 23-Oct-2023, Manuscript No. jnfs-23-28179 (R); Published: 30-Oct-2023 , DOI: 10.35248/2155-9600.23.13.049
Copyright: © 2023 Kedar N Prasad. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.