Journal of Geography & Natural Disasters
Open Access
ISSN: 2167-0587
ISSN: 2167-0587
Research Article - (2023)Volume 10, Issue 2
Since 1970 in Moscow State University (MGU) and after that in 2002, in the Federal (UFPR) and the Federal Technological Universities (UTFPR) of Curitiba (Brazil) have been conducting by the author of this article extensive research on the development of new methods (compositions and technologies) for the disposal of about 90 different types of industrial and municipal waste. The objectives of studies are: 1) Prevent natural and industrial disasters due to the irreversible destruction of natural links by numerous mining quarries and by dumps of all types of industrial, municipal, marine and ocean cleaning waste; 2) Use all of them as valuable raw materials and thus substitute natural raw materials (clay, sand, crushed stone, gravel, etc.); 3) Study the chemical interaction of the initial components and of the developed composite materials structure formation processes; 4) Chemically bind all heavy metals and other hazardous elements into insoluble sustainable compounds; 5) Achieve high mechanical and physical properties of materials with a maximum content of waste; 6) Increase economic efficiency of new composites from waste to make them most attractive for widespread use at an industrial level. All these goals achievement should improve the planet's inhabitants' life quality and should prevent the death of our civilization, predicted by the convincing calculations of S.W. Hawking.
Industrial; Municipal waste utilization; Natural materials substitution; Cement less concrete production; Physical-chemical properties; Sustainable materials; Disasters prevention; Environmental efficiency
Through all modern methods of communication, one can see an increase in the awareness of the high contribution of industrial and municipal waste to the increase of the earth's serious environmental problems. Billions of tons of different waste contaminate the air, land, surface water and groundwater of every country in the world. The steady increase in atmospheric temperature become the most dangerous factors for the survival of our civilization over the next 300 years. The principal reason of this disaster is atmosphere pollution by clogging with gas and dust of industrial and municipal dumps. The insufficient collection and inappropriate disposal of solid wastes leads to water, land and air pollution, and pose risks to human health and the environment. The infamous technological catastrophes are constant in different scales in different parts of the world. The most famous among them as the rupture of the aluminum ore sludge in Hungary and two iron ore dams in Brazil. They happen frequently and on an increasingly high scale with greater numbers of victims and economic loss. The only way to prevent such catastrophes is to stop storing tailings and start recycling them completely as valuable raw materials. All wastes applied as raw materials under the guidance and constant participation of the author, listed below, were divided into ten below listed groups [1]
Research objectives
• Assist to industrial enterprises to solve their environmental problems.
• Develop new composites of materials with maximum percentage content of industrial and municipal wastes.
• Develop methods for the transfer of heavy metals of wastes in practically insoluble environmentally clean new formations, much below of the national standards’ demands.
• Study physical and chemical processes of new materials structure formation to enable control of their properties.
• Develop new or adapt existing technologies of new materials production.
• Develop materials and technologies of its productions with a very high economic efficiency.
Based on our researches, all of the listed below 89 types of industrial and municipal waste can be used to produce the following materials:
• Cement less concrete;
• Conventional ceramics;
• Refractory ceramics;
• Bonding materials (Portland or lime cement type) for the production of road bases, airports; municipal and industrial landfill bases, dam core, etc.;
• Thermal and acoustic insulation;
• New types of fuel with high calorific value;
• Compositions with plastics;
• Decorative materials.
List of industrial and municipal waste under study
To date, the methods use the following types of industrial wastes;
Metallurgical wastes:
• All types of ferrous slag as binders (blast-furnace, Martin, converter, electric-steel, etc.);
• All types of slags of non-ferrous metallurgy (Ni, Al, Cu, Zn, etc.);
• Electric-Ark Filter Dust (EAFD)-1,2,8;
• Dust of non-ferrous metals (Pb, Ni, Zn, Cr, V, Fe, etc.)-1,3,8;
• Heavy metal sludge-1,2,8.
Machin production wastes:
• Residues generated from the processes of the automobile industries: alkaline liquids, dust, pastes - 1,2,3;
• Foundry sands and slags-1,2,3;
• Galvanic processes sludge-1,2;
• Acid battery neutralizing salts-1,2;
• Wet and oily rejects of the grinder-1,2;
• Rejected diatomite with high oil contamination and with galvanic slurry-1;
• Aluminum anodizing slurry-1,2,3;
• Alkaline liquid aluminum anodizing waste-1, 2, 3;
• Slags from the lead recovery process of automotive batteries-1,2;
• Printed circuit manufacturing sludge-1, 2.
Municipal wastes:
• Sludge of municipal sanitation stations-6;
• Ash of sludge burning of municipal sanitation stations-3,4;
• Sewage sludge of municipal water treatment plant without burning and without • Portland cement-1,3;
• Sea-water desalination sludge-1,2;
• Sludge dredging of sediments of ports and rivers without burning and without Portland cement 3;
• Ashes and slag from municipal wastes incinerators-1,2,3;
• Municipal garbage gasification ashes-1,3;
• Residual sludge from laundries-1,3;
• Tannery rejects-1,2;
• Slurry sludge from municipal landfills-1,2;
• Soil mixed with municipal landfills slurry-1,2;
• Ash of municipal landfill slurry burning-1,2,3.
Construction and demolition wastes:
• Lime-producing wastes-1;
• Cement plant waste-3;
• Construction and demolition debris-1;
• Rejection of production and use of mortars-1,2;
• Concrete waste-3;
• MDF production sludge-1;
• Glass waste (blasting dust, glass shreds, electric glass insulators and others)-1,2;
• Porcelain waste (tableware, porcelain electrical insulators, etc.)-1,2,8;
• Portland cement and asbestos cement tiles (Eternite)-1,2,3.
Pulp, paper and cardboard industry wastes:
• Cellulose production slurry-1,2,3;
• Ash and Lime sludge from paper production-1,3;
• Sludge of different types of paper ink-1,2,3;
• Rejected sludge from cardboard production and reuse-1,2,6;
• Paper production sludge-3;
• Toner dust waste-1,2
The ore mining and processing industry wastes:
• Residual soils from mine roofs, clayey soils, etc.-1,3;
• Dredging sludge from canals and sea ports-3;
• Rock waste with high heavy metal content-1,2;
• Rejects of cutting, crushing, sifting and the remains of natural rocks (granite, marble, slate, serpentinite, etc.)-3;
• Weathered rocks of mines-1,2;
• Rejects of extraction of minerals (gold, diamonds, etc.)-1,2;
• Mineral pickling soil -1;
• Purification sludge from mines with high salt and mineral contents-1,2;
• Sawdust and wood shavings-6,7;
• Cuts and powder of asbestos rocks-1,2,3;
• Acidic waste from Jarosite-1,2;
• Alkaline red clay" of Bauxite from the Amazon and Spain-1,2,3;
• Aluminum anodizing slurry-1, 2;
• Liquid waste from the of aluminum production (Spain) -1,2;
• Hydrated gypsum waste-1,8;
• Phosphorus-gypsum waste-1,8;
• Fine and ultrafine mineral coal powder-6;
• Charcoal powder-6;
• Iron ore tailing materials-1,3;
• Overburden soils of various fields-1,3.
Petrochemicals wastes:
• Oily waste (as oily sludge)-6;
• Liquid alkaline wastes from the petrochemical industry-3;
• Rejections of catalysts of petroleum refineries-1,2;
• Oil spilled on soils-6;
• Fine refuse of oil shale-6;
• Burning ash from spilled oil soils-1,2,3.
Energy production wastes:
• ETA and ETE sludge from thermoelectric power plants-1,2;
• Ash of oily shale-1,2,3;
• Mix of ash with poorly burned wood-6;
• Ash from wood burning, bituminous shale, coal, etc.-1,2;
• Waste from the rectification process of metal-mechanical industries-1,2;
• Boiler cleaning carbonates sludge from thermoelectric power plants-1;
• Ashes from the gasification processes of organic materials-6.
Chemical industry wastes:
• Sludge from chemical industries: pastes, powders, alkaline liquid effluents-1, 2;
• Rejects of rigid polyurethane foam from refrigerators, freezers, etc.-5;
• Extrusion of aluminum sulphate-1,2;
• Rejected production of soda ash-1,2;
• Industrial sludge from of different chemical production-1,2;
• Sludge of phosphoric fertilizers and nitrogen production-1;
• Detergent production sludge-1;
• Sludge for the production of cosmetics and perfumery (shampoos, conditioners, soaps, lipsticks; deodorants, beauty and depilatory creams, toothpastes, sun blockers, skin protectors against insects, etc.)-1;
• Ethanol alcohol production catalyst sludge-1,2.
Agrarian complex:
• Ash of husks and stems burning (coffee, rice and other cereals, coffee, sugar cane, etc.-1,2,3;
• Animal waste after methane emission-6;
• Ash after animal wastes incineration-6;
• Non-certified coffee-6.
This experience greatly facilitated the development of other industrial wastes utilization methods.
Example of industrial waste utilization for cement less concrete production
The particle size distribution of the raw materials was executed by laser diffraction analysis on a CILAS 1064 granulometer. The chemical composition determination was conducted by X-rays fluorescence method, using a spectrometer of Philips/ panalytical, model PW 2400. The mineralogical composition of the raw materials and ceramics was studied by powder method in a Philips X-rays diffract meter, model PW1830, at a 2Ï´° range of 2°C-70°C with monochromatic wavelength λ Cu-Kα; the results were interpreted with super-Q X'Pert high score software (database PDF-2). To characterize the morphological structures, it was used Scanning Electron Microscopy (SEM), model FEI Quanta 200 LV. The microchemical analyses were carried out by the Energy Dispersive Spectroscopy method (EDS) in oxford equipment (Penta FET-125 Precision). The isotopic compositions of the new formation were performed by LAMMA-1000 laser micro-mass analyzer, model X-ACT. The comparison of the environmental impact of the raw materials and the developed composites was performed by metals solubility and lixiviation from liquid extracts (NBR 10,004, 2004), using the Atomic Absorption Spectroscopy (AAS) method in a Perkin Elmer 4100 spectrometer. The axial resistance strength of the developed ceramics test samples was controlled in the universal testing machine EMIC DL10,000. Linear shrinkage was determined with a digital caliper of DIGIMESS and Water Absorption (WA) after test samples complete immersion in water for 24 hours. Water absorption and density of the test samples were also studied [2].
Calculations
Flexural resistance of the ceramics was calculated using the formula:
RF=((3 × c × d)/(l × h2)) eq. 1
Where,
Rf=flexural resistance (MPa);
c=maximum load reached at the moment of rupture (kgf) adjusted with a speed of 0.5 mm/min;
d=distance between the supports of the test piece (mm);
l=width of the test piece (mm); h=height of the test piece (mm).
The Coefficient of Water Resistance (CWR) was determined on the 28th and 90th days of cure:
CWR=RWS/RD eq. 2
Where,
RWS-the axial compressive strength of water saturated test samples after total immersion in water for 24 hours,
RD-the axial compressive strength of oven-dried test specimens at 100°C for 24 hours.
Water Absorption (WA, %) values were calculated under NBR 9778, using the equation:
WA=((Ms-MD)/MD) × 100 eq. 3
Where,
MD-sample mass after 24 hours oven drying at a temperature of 110°C (g),
Ms-sample mass after 24 hours water immersion at room temperature (g).
The values for the apparent density DA (g/cm³) of the test samples were calculated using the mass/volume ratio
D=M/V eq. 4
Where,
M=test sample mass (grams);
V=test sample volume (cm³).
The study of the linear shrinkage LS (%) values was conducted in accordance with the equation:
LS=Li=((Li-Ls)/Li) × 100 eq. 5
Where,
Li-initial length of specimen (mm),
Ls-length of the specimen after the sintering (mm).
Bibliographic revision
The largest material intensive consumer of natural materials are cementless concrete and ceramics construction materials. Therefore, a large amount of our research is focused on the application of industrial wastes for the development of cementless concrete and ceramics from different types of waste without natural components or at least with minimal its content. As an example of concrete from industrial wastes this article provides the composition and properties of concrete from four types of wastes, namely from Iron Ore Processing Waste (IOW) with paper and cellulose Pulp Waste (PCW), concrete production and demolition waste (CW) and Lime Production Waste (LPW), without the application of any traditional natural inputs, such as sand, gravel, rubble, because the quarries irreversibly damage the environment [3].
More than 1.2 billion tons of IOW have already been stored in the European Union and between 5 billion tons and 7 billion tons per year in emerging countries. In the rupture of Mariana tailings dam of IOW, in 2015, considered one of the biggest environmental disasters in Brazil and the world, more than 35,000,000 m3 of mud were released into the environment and reached the Atlantic Ocean, affecting the whole ecosystem for over 662 km downstream of the Doce River IBAMA. A similar tragedy occurred again in the city of Brumadinho leaving 259 deaths and 11 people missing due to this huge environmental disaster and the economic damage was several billion dollars [4].
For the Institute of Technological Research (IPT) of Brazil, the world reserve of iron in 2016 was near 170 billion tons; during production of one ton of iron is generated near 400 kg of IOW sludge (i.e., annually about 289 million tons of IOW) with exceptionally low level (5.4%) of utilization. The calculations of Freitas, et al., demonstrated even worse results-only 0.003% IOW are reused. Therefore, between the years 2000 and 2017 there were 36 cases of IOT tailings dams’ failures in the world, an average of two per year, which caused significant environmental damage [5].
The pulp and paper mills also generate a wide variety of organic and inorganic residues, becoming a primary environmental concern as an amount of about 300 kg of sludge is generated for every ton of paper produced. Global paper and cardboard production reached approximately 419,7 million tons in 2017 which means that approximately 126 million tons of highly alkaline waste are discharged into industrial dumps every year, increasing and creating serious environmental problems for our planet. They can be used for different goals, such as clay based brick production, as low-cost substrates with a primary focus on wastes from agro, food, brewery, sugar industries, lignocellulosic biorefineries, and textile and pulp mills [6].
In this study, three main types of waste from the last stage of the Kraft caustification process of paper and pulp production were used, called Dregs, Grits, and Lime mud. For our experimental study they were mixed in the proportion of their production at a local factory near the city of Curitiba. Some authors reported CW production's level until 40% of the worldwide industrial and municipal waste generation. The total amount of waste generated in the European Union in 2010 was over 2.5 billion tons, of which almost 35% (about 860 million tons) came from construction and demolition, with the recycling of up to 46% in 2018 (European commission). Informed that annual CW production in China is about 3 billion tons. The global annual consumption of natural aggregates was around 15 billion tons. CW are inert materials, such as concretes with almost 90% of fragments of natural rocks, mortars, and ceramics, slate, glass, which have proven to be a substitute for natural aggregates. Therefore, it is used partly as a substitute for natural stone material [7].
According to Dowling et al., lime is one of the most versatile chemicals in the world. The 2019 statistic specified the total world lime production at 430 million tons. In agreement with the Brazilian norm NBR 6453, when the content of CO2 and pollutants in lime exceeds 12%, the burned material cannot be sold to the consumer as virgin lime and must be called Lime Production Waste (LPW). It is used mainly to neutralize acid soils, kill bacteria in municipal sanitary sludge, to produce Portland cement clinker [8].
Raw materials characterization
Particle size distribution of the raw materials: The particle size distribution of the studied wastes was classified as fine grains by the sieve method, through the material's flow in a mesh of 4.76 mm to 250 μm. The particles with sizes from 180 μm to 1 μm were analyzed in a Cilas 1064 laser granulometer. The main part of the IOW (77.89%) was 1.68 mm-2.38 mm in size (Tables 1 and 2), with a specific mass of 3.280 kg/m3, and a pH value of 10.21 [9].
Concrete Waste (CW) consisted mainly (87.8%) of the particle sizes between 4.76 mm-2.00 mm with a specific mass of 2.280 kg/m³ and pH=10.34. The LPW's distribution curve showed two peaks with high particle size content: The first one between 1.68 mm-2.38 mm with 43.4% of particles, and the second one with 37.6% of particles at 1.19 mm. LPW specific mass was 1.250 kg/m³ and pH=11,00. The maximum volume (65.98%) of CW particles had a diameter 0.010 mm-0.028 mm with pH=11.62 [10].
| Particles' size, mm | Particles' size distribution,wt. % | ||
|---|---|---|---|
| IOW | CW | LPW | |
| 4.76 | 0.6 | 9.9 | 1 |
| 4 | 0.4 | 9.3 | 1.2 |
| 3.36 | 5 | 8.5 | 5 |
| 2.83 | 6 | 4 | 6 |
| 2.38 | 25.3 | 45.9 | 15 |
| 2 | 38 | 10.2 | 12.2 |
| 1.68 | 16.6 | 1.5 | 16.2 |
| 1.41 | 3.1 | 1.5 | 3.1 |
| 1.19 | 2.3 | 1.4 | 37.5 |
| 0.59 | 1 | 1.9 | 1 |
| 0.3 | 0.9 | 0.8 | 0.5 |
| Bottom | 0.7 | 5 | 1.3 |
| Total | 99.9 | 99.9 | 100 |
Note: IOW: Iron Ore Waste; CW: Concrete Waste; called Lime Production Waste (LPW)
Table 1: Particles' size distribution of the iron ore waste, concrete waste, and lime production waste.
| Particles' size, mm | Particles' size distribution, wt. % |
|---|---|
| 2 | 0.3 |
| 0.5 | 0.2 |
| 0.25 | 2.6 |
| 0.063 | 0.07 |
| 0.043 | 5.33 |
| 0.036 | 8.04 |
| 0.028 | 16.59 |
| 0.02 | 21.13 |
| 0.01 | 28.26 |
| 0.006 | 7.76 |
| 0.003 | 4.78 |
| 0.001 | 3.61 |
| Bottom | 1.31 |
| Total | 98.69 |
Table 2: Particles' size distribution of Paper and Cellulose production Wastes (PCW) particles' size, mm particles' size distribution.
Chemical composition of the raw materials under study
The chemical composition of IOW (Table 3) consisted mainly of Fe2O3 and SiO2 (sum 90.0%), CW of CaO and SiO2 (sum 71.6%), LPW of CaO and MgO (sum 84.0%), and PCW of CaO (52.3%) with an extremely high calcination loss (C.L.=41.3%) due to the high carbonates content. For the same reason, C.L. was 16.2% for LPW and 10.6% for CW [11].
| Elements | IOW | CW | LPW | PCW |
|---|---|---|---|---|
| Fe2O3 | 48.1 | 1.9 | 0.7 | 0.4 |
| SiO2 | 41.9 | 49.1 | 1.9 | 1.7 |
| Al2O3 | 6.3 | 6.4 | 0.5 | 0.5 |
| MgO | 0 | 3.1 | 30.6 | 1.4 |
| K2O | 0.2 | 1.7 | <0.1 | 0.3 |
| Na2O | 0 | 1.5 | 0 | 0 |
| CaO | 0.2 | 22.5 | 49.8 | 52.3 |
| MnO | <0.1 | 0 | <0.1 | <0.1 |
| SO3 | <0.1 | 0.9 | <0.1 | 1.1 |
| TiO2 | 0 | 0.7 | 0 | <0.1 |
| P2O5 | 0 | 0.5 | 0 | 0.3 |
| P.F. | 3.1 | 10.6 | 16.2 | 41.3 |
Note: IOW: Iron Ore Waste; CW: Concrete Waste; called Lime Production Waste (LPW)
Table 3: Chemical composition of the raw materials.
Mineral composition of the raw materials
Mineral composition of Iron Ore Waste (IOW, Figure 1) comprised three iron oxides-hematite Fe2O3, magnetite Fe3O4, and goethite FeO (OH), the mineral of clay kaolinite Al2Si2O5 (OH)4, and, the most common mineral in the Earth's crust, quartz SiO2. All mineral peaks had an extremely low intensity, except for one peak of quartz at the angle of 2θ°=26.8° and one common peak of hematite and magnetite, which coincided at an angle of 2θ°=27.2°. The intensity of both maximum peaks was extremely low about 200 Cps (counts per second); all the remaining peaks only slightly exceeded the X-ray background, indicating either a massive dissociation of their crystal lattices due to the mechanical and chemical processes of iron ore processing or due to the rapid cooling of this volcanic lava during its outflow. XRD pattern of concrete waste showed the presence of the not fully hydrated mineral of Portland cement carbonate brownmillerite (4CaO.Al2O3.FeO3), portlandite Ca(OH)2, calcite CaCO3 and dolomite CaMg(CO3)2, and quartz SiO2. Mineral composition of LPW confirmed the correct classification of this material as a waste of lime production since no lime crystal lattice peaks were found regardless of concurrence with other minerals. Simultaneously, the most intense peaks are those of portlandite (as hydrated lime) and calcite and dolomite (as portlandite, which reacted with atmospheric CO2) [12].
The Cellulose Production Wastes (CPW) showed the highest crystallinity level among the raw materials since the highest calcite peak at 2θ°=29.5° reached 3000 cps and many other peaks approached 500 cps. PCW included calcite CaCO3, dolomite CaMg(CO3)2, pirssonite Na2Ca (CO3)2.2H2O, and quartz SiO2 [13].
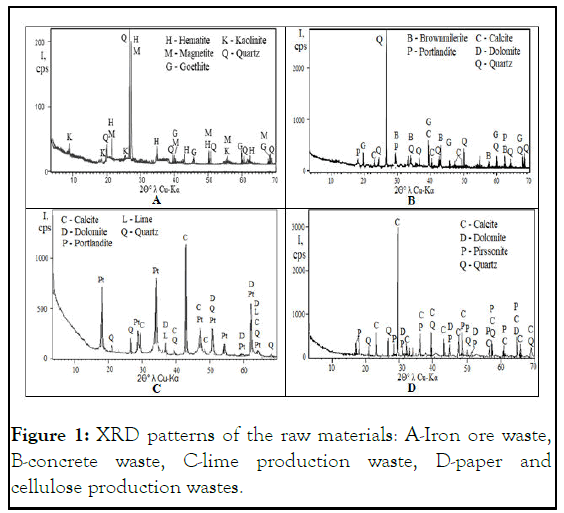
Figure 1: XRD patterns of the raw materials: A-Iron ore waste, B-concrete waste, C-lime production waste, D-paper and cellulose production wastes.
Morphological microstructures of the raw materials
Almost all particles of the raw materials, studied by the SEM method at the micro-level (Figure 2), presented were in the form of separate spherical or irregular rounded particles or its adhering aggregates. Only PCW particles were represented by fragments of crystal-like combinations of prism and pyramid forms. Inside these cellulose crystals, channels of different diameters, characteristic of the wood structure, were neatly visible [14].
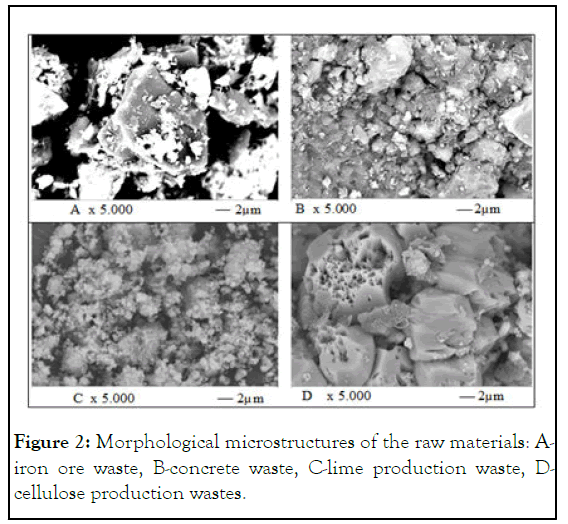
Figure 2: Morphological microstructures of the raw materials: Airon ore waste, B-concrete waste, C-lime production waste, Dcellulose production wastes.
Mechanical and physical properties of developed materials
In order to study the feasibility of using the developed compositions as construction materials, the changes in their main properties were investigated throughout the hydration and hardening process of the test samples, particularly, their axial resistance, linear expansion, and shrinkage, water absorption, and water resistance [15].
Axial resistance of the test samples
Composition 7 (Table 4), with almost maximum IOW (40%) and minimum PCW content (10%), showed the maximum resistance values at all ages, followed by compositions 8, 6, and 5. The 30% difference in IOW and PCW amounts between compositions 6 and 7 or 25% between compositions 7 and 8 demonstrated IOW's beneficial role regarding PCW. This conclusion was corroborated by comparing the resistance of compositions 1 and 2, or 3 and 4, or compositions 1 and 3, or 2 and 4-samples with higher IOW content with equal amounts of other components, always exhibited higher resistance. [16].
| N° | Compositions, % | Axial resistance of composites (MPa)/days of cure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IOW | PCW | CW | LPW | 3 | 7 | 14 | 28 | 60 | 90 | 180 | 365 | 720 | |
| 1 | 20 | 40 | 25 | 15 | 1,20 | 2,10 | 2,31 | 2,44 | 2,71 | 2,90 | 3,15 | 4,93 | 5,41 |
| 2 | 40 | 20 | 25 | 15 | 1,83 | 2,67 | 2,75 | 2,86 | 2,93 | 3,03 | 3,80 | 5,06 | 6,26 |
| 3 | 15 | 45 | 25 | 15 | 1,40 | 1,70 | 1,67 | 1,56 | 1,54 | 2,15 | 3,52 | 5,17 | 5,17 |
| 4 | 45 | 15 | 25 | 15 | 2,62 | 3,40 | 3,45 | 3,61 | 3,88 | 4,43 | 4,78 | 5,70 | 6,90 |
| 5 | 40 | 10 | 30 | 20 | 2,84 | 3,93 | 4,00 | 4,41 | 3,64 | 4,82 | 6,59 | 6,85 | 7,06 |
| 6 | 10 | 40 | 25 | 25 | 3,00 | 3,34 | 3,75 | 4,07 | 4,32 | 4,79 | 5,87 | 6,97 | 7,91 |
| 7 | 40 | 10 | 25 | 25 | 4,94 | 5,75 | 6,58 | 6,92 | 7,61 | 7,72 | 9,63 | 10,47 | 11,47 |
| 8 | 15 | 35 | 25 | 25 | 3,26 | 3,58 | 3,81 | 4,35 | 4,44 | 4,85 | 5,98 | 7,02 | 8,38 |
Note: IOW: Iron Ore Waste; CW: Concrete Waste; called Lime Production Waste (LPW)
Table 4: Changes in axial resistance of composites during hydration and cure with equal amounts of other components, always exhibited higher resistance.
The IOW content reduction in composition 6 compared to compositions 1 and 5 was offset by the increase in LPW to 10% and 5%, correspondingly. The maximum resistance of the 7-dayage samples reached 5.75 MPa. The positive role of the LPW was especially evident when replacing 10% PCW with an equal amount of LPW in compositions 2 and 7, leading to an almost double increase in resistance at 720 days (11.47 versus 6.26 MPa). The compositions' resistance values increased with the curing time and directly proportional to LPW and IOW contents [17].
According to the Brazilian standard NBR 7170, solid bricks are classified into three classes: Class A>1.5 MPa; Class B>2.5 MPa; and Class C>4.0 MPa. On the third curing day, the resistance significantly exceeded the demands for Class A, on the 28th day; four compositions have beaten the demand for Class C. At the age of 1 year, all compositions considerably surpassed the requirements for solid bricks Class C [18].
Coefficient of Water Resistance (CWR) of the Test Samples (TSs)
The water resistance values of the TSs (Table 5) were calculated using equation 2. The analysis of the study results showed an increase in CWR with the hydration and hardening timefrom 28 days to 90 days [19].
| N° | Compositions, wt. % | Coefficient of water resistance of the materials | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| on the 28th day of cure | on the 90th day of cure | |||||||||
| IOW | PCW | CW | LPW | RDRY | RHUMID | CWR | RDRY | RHUMID | CWR | |
| 1 | 20 | 40 | 25 | 15 | 2.44 | 1.46 | 0.6 | 2.9 | 1.8 | 0.62 |
| 2 | 40 | 20 | 25 | 15 | 2.86 | 1.77 | 0.62 | 3.03 | 2 | 0.66 |
| 3 | 15 | 45 | 25 | 15 | 1.56 | 0.86 | 0.55 | 2.15 | 1.23 | 0.57 |
| 4 | 45 | 15 | 25 | 15 | 3.61 | 2.41 | 0.67 | 4.43 | 3.1 | 0.7 |
| 5 | 40 | 10 | 30 | 20 | 4.41 | 3.13 | 0.71 | 4.82 | 3.3 | 0.73 |
| 6 | 10 | 40 | 25 | 25 | 4.07 | 3.22 | 0.79 | 4.79 | 3.93 | 0.82 |
| 7 | 40 | 10 | 25 | 25 | 6.92 | 6.37 | 0.92 | 7.72 | 7.26 | 0.94 |
| 8 | 15 | 35 | 25 | 25 | 4.35 | 3.13 | 0.72 | 4.85 | 3.49 | 0.72 |
Note: IOW: Iron Ore Waste; CW: Concrete Waste; called Lime Production Waste (LPW)
Table 5: Water resistance (CWR) of the materials after 28 curing days and 90 curing days.
As the increase in resistance, the dependence of the materials' CWR with the content of the component used as a binder (LPW) and as a compaction agent (IOW) was plainly visible. Therefore, the highest CWR values were detected for composition 7 on the 28th day and 90th day (0.92 and 0.94, correspondingly), followed by composition 6 with CWR=0.79 and 0.82 and compositions 5 and 8. Composition 3 exhibited the lowest CWR values (CWR=0.55 and 0.57), followed by composition 1 (CWR=0.60 and 0.62) and composition 2 (CWR=0.62 and 0.66) [20].
The maximum allowable decrease in resistance for TSs fully saturated with water on the 90th day is 35% for class 3; 30% for class 2 and 20% for class 1. Therefore, regarding water resistance, composition 2 met the requirements of class 3, compositions 4, 5, and 8-of class 2 and compositions 6 and 7 class 1.
Water Absorption (WA) of the TSs
The Water Absorption (WA) values (Table 6) were determined by equation 3 on the 28th day and 90th day of cure. The reduction regularly observed on the 90th day was due to the new formations that started filling the pore space of the TSs. The lowest results were shown by composition 7 with WA=9.62 and 8.33 on the 28th day and 90th day correspondingly, followed by compositions 5, 6, and 8. This fact might be the best explication for their best axial and water resistance values [21,22].
| N° | Composition (wt. %) | Water absorption (wt. %) | ||||
|---|---|---|---|---|---|---|
| IOW | PCW | CW | LPW | 28 days | 90 days | |
| 1 | 20 | 40 | 25 | 15 | 13.59 | 12.25 |
| 2 | 40 | 20 | 25 | 15 | 12.53 | 11.93 |
| 3 | 15 | 45 | 25 | 15 | 14.93 | 12.72 |
| 4 | 45 | 15 | 25 | 15 | 11.39 | 10.85 |
| 5 | 40 | 10 | 30 | 20 | 10.11 | 9.17 |
| 6 | 10 | 40 | 25 | 25 | 10.36 | 9.3 |
| 7 | 40 | 10 | 25 | 25 | 9.62 | 8.33 |
| 8 | 15 | 35 | 25 | 25 | 10.84 | 10.09 |
Note: IOW: Iron Ore Waste; CW: Concrete Waste; called Lime Production Waste (LPW)
Table 6: Water absorption of the TSs on the 28th day and 90th day of cure.
NBR 6136 establishes a WA value between 10% and 16% as average WA values for concrete sealing blocks.
Coefficient of expansion (CEXP) and shrinkage of the materials
In the test samples' hydration and hardening process, there were two antagonistic processes- expansion up to 7 days inclusive, followed by their shrinkage up to 720 days (Table 7). It was observed and investigated in detail this transition from expansion to shrinkage at different ages of samples up to 60 days and even 90 days, depending on the chemical and mineral composition of raw materials. Both phenomena are explained initially by the active sol synthesis in the samples' porous space due to the alkaline corrosion of the solid particle surfaces and their volume expansion by these new formations (in this case, up to 7 hydration days), followed by the transition from sol to gel. Between 7 days and 14 days, the gel's aging process (syneresis) has begun, expressing itself by its compaction with a decrease in the TSs volume up to 720 days or more [23].
| N° | Compositions, wt. % | CEXP (%) of the TSs during hydration and cure (days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IOW | PCW | CW | LPW | 3 | 7 | 14 | 28 | 60 | 90 | 180 | 365 | 720 | |
| 1 | 20 | 40 | 25 | 15 | 2.45 | 3.34 | 3.32 | 2.18 | 1.7 | 1.62 | 1.36 | 1.06 | 0.96 |
| 2 | 40 | 20 | 25 | 15 | 2.47 | 3.44 | 3.38 | 2.2 | 1.9 | 1.73 | 1.32 | 1.12 | 0.97 |
| 3 | 15 | 45 | 25 | 15 | 2.32 | 3.28 | 3.22 | 2.21 | 1.78 | 1.64 | 1.28 | 1.11 | 0.96 |
| 4 | 45 | 15 | 25 | 15 | 2.48 | 3.22 | 3 | 2.2 | 1.89 | 1.73 | 1.32 | 1.05 | 0.84 |
| 5 | 40 | 10 | 30 | 20 | 2.65 | 3.4 | 3.32 | 2.54 | 1.6 | 1.38 | 1.43 | 1.22 | 0.93 |
| 6 | 10 | 40 | 25 | 25 | 2.78 | 3.55 | 3.4 | 2.24 | 1.68 | 1.44 | 1.16 | 1.05 | 0.98 |
| 7 | 40 | 10 | 25 | 25 | 3.2 | 3.92 | 3.65 | 2.43 | 1.32 | 1.24 | 1.15 | 1.12 | 1.08 |
| 8 | 15 | 35 | 25 | 25 | 2.76 | 3.57 | 3.48 | 2.23 | 1.95 | 1.57 | 1.34 | 1.15 | 0.96 |
Note: IOW: Iron Ore Waste; CW: Concrete Waste; called Lime Production Waste (LPW)
Table 7: Linear expansion and shrinkage of materials during the cure.
The most considerable magnitude of expansion (CEXP=3.55-3.92%) in 7 days was observed for compositions 6-8 with the highest chemically active LPW content (25%), and, among them, composition 7 with 40% IOW (CEXP=3.92%). These values confirmed once more the dependence of the properties of the materials on the amount of binder LPW and the IOW. Compositions 1-4 exhibited significantly lower CEXP values because they contained 15% LPW, and, therefore, lower alkalinity of the hydrated initial mixes with less quantity of sol formation. On the 720th day, composition 7 remained the one with the highest CEXP=1.08%, and composition 4 the one with the lowest CEXP=0.84%, followed by compositions 3 and 1 (CEXP=0.96%) [24].
Physical-chemical processes of the structure's formation
To better explain the results obtained for the mechanical and physical properties and their changes during the 720 hydration days, composite 7 was chosen, as it presented the best values at all ages of curing, thus manifesting the processes more intensely, making it easier to observe. Besides, composite 7 held almost the highest IOW content (40 wt. %), the most contaminant component, and, therefore, the most important to prevent environmental impact [25].
Changes in mineral compositions during materials hydration
Mineral composition of the composite 7 after 3 hydration days (Figure 3) consisted of portland cement mineral brownmillerite Ca2(Аl,Fe)2O5, hydrated lime mineral portlandite Ca(OH)2, calcite CaCO3, clay mineral kaolinite Al4(Si4O10)(OH)8, three iron ore waste minerals-hematite Fe2O3, magnetite Fe3O4 and goethite FeO(OH) and quartz SiO2. The XRD pattern also had a rather high X-ray background, indicating a large amount of amorphous phase due to both geological and industrial processes that occurred with each of the components. Therefore, the crystalline structures of all minerals were extremely destructed, and the intensities of their peaks were very low. Only one of the quartz crystalline peaks exceeded 2,000 cps (counts per second) [26].
During the 180 days of composite 7 hydration in a highly alkaline environment, the complete destruction of kaolinite and portlandite weak crystal lattices occurred. Despite the samples expansion by the order of 3.92%, the samples of composite 7 hardened to 4.94 MPa. Only the calcite peak significantly increased at 2θ°=29.5°, but it is not able to explain such increase in resistance [27].
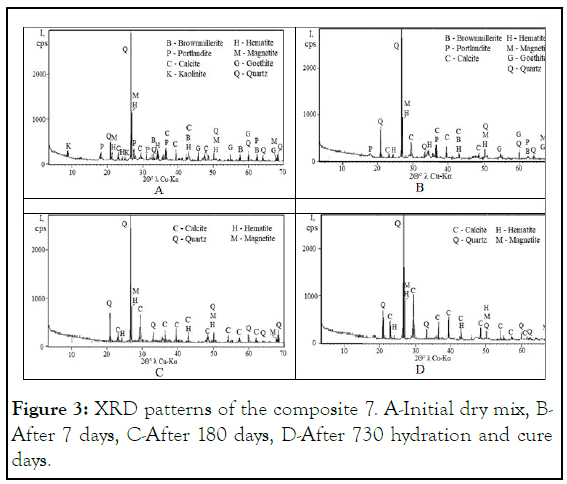
Figure 3: XRD patterns of the composite 7. A-Initial dry mix, BAfter 7 days, C-After 180 days, D-After 730 hydration and cure days.
On the 720th, the material resistance doubled (up to 10.47 MPa) due to the complete hydration of portlandite, brownmillerite, and goethite. The chemical dissociation of these minerals in the form of new amorphous formations in the test samples' pores led to a decrease in water absorption (Table 6) and an increase in water resistance [28].
Such phenomena could only happen thanks to the synthesis of a significant amount of sol from the dissociation of kaolinite, brownmillerite, portlandite, and goethite in an alkaline environment, and the sol transformation and compaction to the gel state with completion of the linear expansion process and the continuity of the almost six-month shrinkage process of the samples from 3.57% to 1.34% (Table 7). There was also the highly visible emergence of new calcite peaks at angles 2Θ°=23.2°, especially 29.5°, 43.5°, 48.5°, 54.0°, 57.5°, and 61.5°. The synthesis of new calcite bodies or the improvement of their structure increased significantly during the 720 days of test samples hydration compared to 180 days. Such a new crystalline phase amount in the materials porous space might become a significant factor in the enhancement of all the above studied properties, along with the ongoing solidification of the amorphous phase [29].
Morphological microstructures of the TSs at 365th day of curing
The images used to study the changes in the microstructure of the samples of composition 7 during their hydration and curing, carried out by SEM with a magnification of 6,000 times, can be seen in Figure 4.
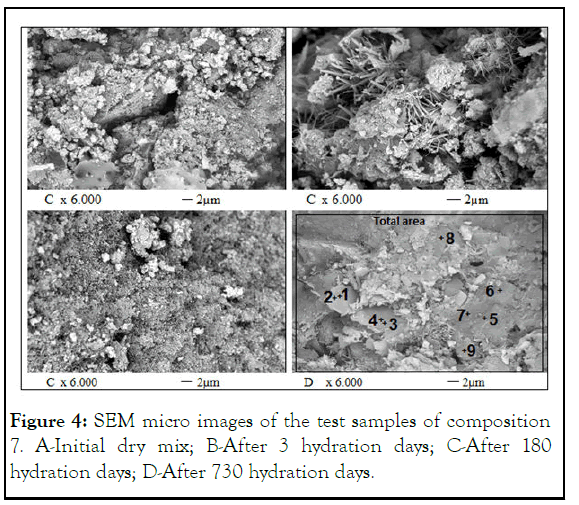
Figure 4: SEM micro images of the test samples of composition 7. A-Initial dry mix; B-After 3 hydration days; C-After 180 hydration days; D-After 730 hydration days.
In the initial dry mix, particles of different shapes with sizes from 0.5 μm to 30 μm were observed. The smaller particles were predominantly rounded while the larger ones were mostly angular. The pores between the particles were also very diverse, both in size and shape. It was seen that the particles were not bonded to each other by any bonding materials and did not have a common structure [30].
After 3 days of hydration and compaction process in an alkaline environment, the appearance of many new needle-shaped formations, druse-like crystals growing from one point is clear. When compared to, there was a noticeable reduction in the number of small particles due to their aggregation. This aggregation process was significantly intensified in samples at 180 days, and large monolithic fields appeared in the samples' structure at 730 days. The vast predominance of such monolithic sections might explain the changes in the most important mechanical and physical properties of the samples at this age, such as water absorption, water resistance, axial resistance, linear expansion, and shrinkage [31].
Micro chemical compositions of the new formations
The study of the microchemical composition of the new formations that strengthen the developed compositions was performed by the EDS method on samples from composition 7 on the 730th day (Table 8). All points, including those closest to each other (1 and 2; 3 and 4), exhibited very different [32].
| Points | C | Mg | Al | Si | Ca | Fe | Cu | Total |
|---|---|---|---|---|---|---|---|---|
| 1 | 22.44 | 25.77 | 0.26 | 0.64 | 36.38 | 10.58 | 2.93 | 100 |
| 2 | 17.9 | 20.4 | - | 1.72 | 37 | 20.51 | 3.47 | 100 |
| 3 | 26.78 | 31.72 | 1.93 | 10.67 | 27.9 | 0.42 | 0.58 | 100 |
| 4 | 31.6 | 5.5 | 15.63 | 6.17 | 29.66 | 9.03 | 2.41 | 100 |
| 5 | 27.69 | 20.72 | 0.42 | 2.22 | 37.62 | 9.33 | 2 | 100 |
| 6 | 20.70 | 9.89 | 3.59 | 1.43 | 41.49 | 21.21 | 1.69 | 100 |
| 7 | 30.65 | 23.61 | - | 0.83 | 36.09 | 8.82 | - | 100 |
| 8 | 16.44 | 20.57 | - | 2.73 | 29.57 | 30.41 | 0.28 | 100 |
| 9 | 26.93 | 30.92 | - | 10.68 | 20.95 | 10.52 | - | 100 |
| Total area | 35.80 | 12.09 | 0.3 | 0.75 | 39.33 | 10.69 | 1.04 | 100 |
Table 8: Chemical composition of the new formations of composition 7 on the 730th day of cure (by the EDS method).
Chemical compositions. None of these points were similar in chemical composition to the total area. Such heterogeneity is due to the practical impossibility of achieving ideal homogeneity at the micro level when mixing dry components before hydration. Therefore, sol formed during alkaline corrosion of different solid particles, which then chemically reacted before turning into a gel, presenting significantly different compositions at the micro-level in this study. Nevertheless, such heterogeneity of the gel did not prevent it from playing a decisive role in the material hardening [33].
Isotopic composition of the new formations
Isotopic composition of the number 7 composite after 720 hydration days was studied by Laser Micro Mass Analyses (LAMMA) (Figure 5). The set of isotopes and their intensity at each of the new formations' six study.
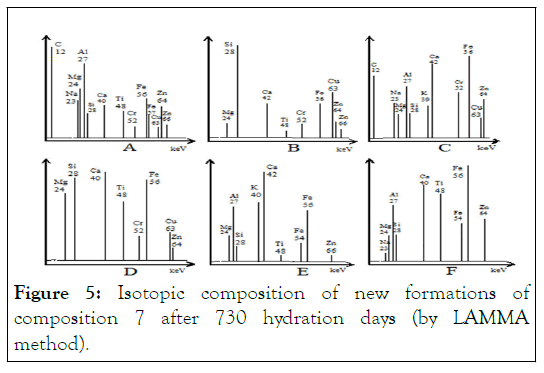
Figure 5: Isotopic composition of new formations of composition 7 after 730 hydration days (by LAMMA method).
Points were significantly different from each method (Table 8). In addition, the presence of heavy metal isotopes (Cr, Cu, and Zn) in most of the plotted points of new formations was established by the LAMMA method [34].
Environmental impact of the developed materials
The recognition of heavy metal isotopes in composite 7 by the LAMMA method made it necessary to study the solubility and leaching of metals in composite 7 and IOW as the only possible source of their appearance (Table 9) [35].
| Elements | Leaching, mg/L | Solubility, mg/L | ||||
|---|---|---|---|---|---|---|
| IOW | Comp. 7 | NBR 10004 | IOW | Comp. 7 | NBR 10004 | |
| Al | 7.24 | 2.18 | * | 3.07 | 0.08 | 0.2 |
| Cr total | 6.21 | 1.23 | 5 | 5.16 | 0.02 | 0.05 |
| Fe | 3.14 | 0.15 | * | 2.11 | 0.15 | 0.3 |
| Cu | 1.26 | 0.38 | * | 1.08 | 0.09 | 2 |
| Cd | 0.03 | 0.05 | 0.5 | 0.11 | <0.005 | 0.005 |
| Pb | 1.17 | 0.08 | 1 | 1.66 | <0.01 | 0.01 |
| Hg | 0.07 | 0.01 | 0.1 | 0.04 | <0.0002 | 0.001 |
| Se | 0.24 | - | 1 | 0.17 | - | 0.01 |
| As | 0.42 | 0.12 | 1 | 0.06 | <0.001 | 0.01 |
| Ba | 11.17 | 0.15 | 70 | 5.14 | <0.1 | 0.7 |
| Zn | 5.18 | 0.48 | * | 4.18 | <0.10 | 5 |
Table 9: Results of leaching and solubility tests of composite 7 after 730 hydration days.
In the initial mixtures. The analyses using the AAS method Table 9 shows that the metal content (Al, Cr, Fe, Cd, Pb, and Zn) in IOW slightly exceeded Brazilian toxicity standards NBR-10,004. The leaching and solubility of composite 7 by the AAS method demonstrated the possibility of decreasing these values far below the NBR 10,004 demands. Therefore, the developed materials are environmentally clean and can be used as natural aggregates at the end of their service life [36].
Economic efficiency of the application of the developed materials
It is impossible to estimate the economic efficiency of using industrial waste without information on the application in specific sites with the local price of natural raw materials, distances and delivery prices, and many other data sources. Therefore, the calculation of economic efficiency was not included in the objectives of this study. Nevertheless, in terms of common sense, free industrial wastes instead of relatively expensive natural materials must be seen as very cost-effective. Also, considering that tailings are materials that have already been extracted, crushed and processed, the real beneficiation costs for their reuse are significantly lower compared to the primary ore tailing that need to go through the entire mining beneficiation process, since processing represents about 40% to 60% of the total mineral processing cost [37].
According to this study's objectives, building materials were developed from four industrial wastes pulp and cellulose production, iron ore processing, concrete production and demolition, and lime production wastes without the use of traditional natural materials. The structures and properties of the industrial wastes used as raw materials were characterized; the changes in the properties of the test samples during hydration and cure were studied, as well as the physical-chemical processes of the developed composites structures formation. The developed materials showed excellent mechanical properties, such as axial resistance, water resistance and water absorption, expansion and shrinkage. The samples' axial resistance reached values until 4.94 MPa after 3 curing days, 5.75 MPa after 7 days, and 11.47 MPa at 720 days of outdoor curing. The water resistance coefficient of composite 7 on the 28th day and 90th day of cure was 0.92 and 0.94, respectively; the water absorption values-9.62%-8.33%; linear expansion on the 90th day reached 1.24% and on 720th-1.08%. All properties of the developed composites met the requirements of Brazilian standards for the production of construction materials, such as road and airfield runways, levee cores, industrial and municipal dumps, building foundations, bricks, and blocks. The results of solubility and leaching studies of the metals in acid solutions confirmed their environmental friendliness.
All raw materials and developed materials were studied by the XRD, SEM, EDS, and LAMMA methods to explain their properties. The sol solution's chemical interaction process, formed during the alkaline corrosion of solid particles' surfaces of the initial components, was investigated. The gradual densification of the sol and its transition into a hardening gel provided the strengthening of the developed materials over time. The only crystalline form increased in number or perfection was the calcite crystal, which was also a positive factor in strengthening the materials' structure. The feasibility of using four types of industrial wastes, currently stored in dumps that poison the environment, to completely substitute traditional natural raw materials, whose careers irreversibly destroy the environment, has been experimentally proven.
• We declare absence any conflicts of interest.
• Funding (information that explains whether and by whom the research was supported).
• Data and material are available.
• Code availability (software application or custom code) not applicable.
• Ethics approval (include appropriate approvals or waivers) not applicable.
• We show informed consent and provide assurances that participants’ rights are protected.
• All co-authors are consent for publication.
Vsevolod Mymrin-author of the ideas, developer of the plan of the experiments, participant of all stages of the research, interpreter of experimental results, corresponding author.
The authors express their sincere gratitude to the staff of the Laboratory of Minerals and Rocks (LAMIR) of the federal university of Parana for technical assistance in conducting analyzes of the chemical and mineral composition of the materials under study.
Citation: Mymrin V (2023) Prevention of Natural and Industrial Disasters by Hazardous Industrial and Municipal Waste Utilization as Valuable Raw Materials with High Economic and Environmental Efficiency. J Geogr Nat Disasters. 13:269.
Received: 14-Nov-2022, Manuscript No. JGND-22-20114; Editor assigned: 16-Nov-2022, Pre QC No. JGND-22-20114 (PQ); Reviewed: 30-Nov-2022, QC No. JGND-22-20114; Revised: 30-Jan-2023, Manuscript No. JGND-22-20114 (R); Published: 06-Feb-2023 , DOI: 10.35248/2167-0587.23.13.269
Copyright: © 2023 Mymrin V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.