
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2023)Volume 13, Issue 2
Introduction: Primaquine-induced methemoglobinemia is a rare pathological condition typically characterized by mild-moderate symptoms driven by an increase in Methaemoglobin (MetHb) levels (normal value <1%), which can lead to primaquine discontinuation, with major consequences for the management of the underlying disease. Primaquine increases MetHb levels through a pro-oxidant environment promoted by its metabolites. To this aim, we tested the hypothesis that N-Acetyl Cysteine (NAC), by acting as a reducing agent, could be safe and effective in the treatment of primaquine-induced methemoglobinemia not requiring methylene blue treatment.
Methods: We report a 41-years-old woman affected by multiple sclerosis, in treatment with immune-modulatory therapy, who was hospitalized for the management of Pneumocystis jirovecii Pneumonia (PJP) treated with primaquine 30 mg/day and clindamycin 1800 mg/day. At the time of admission MetHb was <1% and after nine days of treatment, a mild increase in MetHb levels was observed (9%). The increase in MetHb was ascribed to primaquine, after ruling out other causes, including Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency. NAC-based antioxidant therapy was introduced at the dose of 3600 mg/day (per os) in order to allow second-line primaquine treatment for PJP.
Results: MetHb levels gradually decreased until the physiological range. Given primaquine and its metabolites halflife, NAC therapy lasted one week longer than the duration of primaquine therapy (total 19 days). No adverse drug reaction to NAC therapy has been reported.
Conclusion: NAC-based antioxidant therapy has been proved effective and safe for the treatment of primaquineinduced methemoglobinemia.
Primaquine; N-Acetyl Cysteine (NAC); Methemoglobinemia; Drugs; Methylene blue
Primaquine is an antimalarial drug that works with a not completely clear mechanism which involves the generation of H2O2 by its hydroxylated metabolites [1]. Primaquine is indicated in the treatment of Pneumocystis jirovecii Pneumonia (PJP) in combination with other drugs [2]. Primaquine induces an increased amount of Methemoglobin (MetHb) in the blood, because of its oxidative activity [3-5].
Methemoglobinemia is a rare pathological condition characterized by high MetHb levels [6], a form of hemoglobin in which heme irons are oxidized in the Fe3+ form [6,7]. Unlike the ferrous heme (Fe2+), the ferric one (Fe3+) is unable to bind O2 [6-8]. The inability of MetHb to adequately transport oxygen, combined with the leftward shift of the hemoglobin dissociation curve, could produce symptoms typical of hypoxia [6]. Routinely, MetHb levels are detected by Arterial Blood Gas analysis (ABG) [9], and physiologically range below 1% of total hemoglobin [6].
Many conditions may induce methemoglobinemia; the easiest way to classify them is to distinguish inherited from acquired causes [6,8]. About the latter, MetHb increase derives from exposure to xenobiotics, especially those capable of inducing oxidative stress [6]. Predisposing conditions are partial deficiency of NADH-cytochrome b5 reductase enzyme or chronic diseases such as respiratory failure, lung diseases, heart failure, hematological diseases, kidney failure, liver cirrhosis, and HIV infection [10].
Traditionally, methylene blue represents the gold standard in treating symptomatic methemoglobinemia [6]. Nevertheless, some studies had shown the efficacy of reducing agents (like high-dose vitamin C) in treating methemoglobinemia when methylene blue was not indicated, contraindicated or unavailable [11].
N-Acetyl Cysteine (NAC) is an acetylated cysteine medication that acts as a glutathione synthesis precursor and electron donor [12], possibly reducing primaquine’s toxicity metabolites and the inflammation induced by oxidation. This case report aims to show how NAC could represent a valid strategy in treating asymptomatic cases of mild to moderate primaquine-induced methemoglobinemia.
A 41-years-old woman affected by multiple sclerosis and in treatment with immune-modulatory therapy was hospitalized for the onset of arthralgia, myalgia, intense headache, chest tightness, dry cough, rhinorrhea, and fever (max 37.4°C). Four days earlier she was discharged from the hospital, after a full course of antibiotic treatment, with a diagnosis of PJP inducing acute respiratory failure. She was treated with atovaquone 1500 mg (split into two administrations) because of an adverse reaction to cotrimoxazole.
Upon entering our department, the physical examination revealed a new reduction in the vesicular murmur at the level of the lung bases. The ABG showed the presence of hypoxemia (60 mmHg) with a marked increase in lactates (27 mmol/L); baseline MetHb was<1%. A nasopharyngeal swab performed to rule out possible COVID-19 pneumonia was negative. A contrast-enhanced chest CT scan showed ground glass thickening, strongly suggesting recurrence of PJP. Bronchoalveolar lavage performed during the last hospitalization was not repeated (Figures 1 and 2).
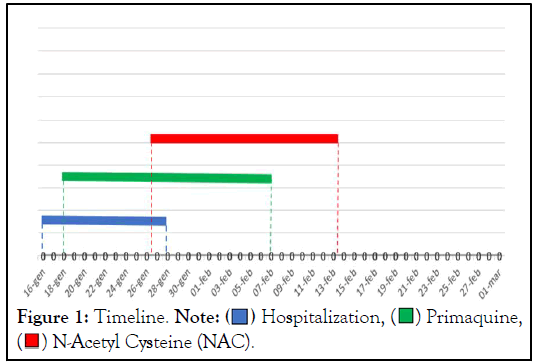
Figure 1: Timeline. Note:  Hospitalization,
Hospitalization,  Primaquine,
Primaquine,  N-Acetyl Cysteine (NAC).
N-Acetyl Cysteine (NAC).
Figure 2: Chronologic observation of patient's MetHb levels and its relation with N-Acetyl Cysteine (NAC) therapy. Note:  NAC, 3600 mg/day,
NAC, 3600 mg/day,  Primaquine, 30 mg/day.
Primaquine, 30 mg/day.
Therefore, due to the clinical failure of the first course of treatment, second-line antibiotic therapy with primaquine base 30 mg/day and clindamycin 1800 mg/day (split into three administrations) was introduced. After nine days of treatment, a mild increase in MetHb levels was observed (9%), in the absence of other signs or symptoms.
The increase in MetHb was ascribed to primaquine, after ruling out other causes, including Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency. Since the administration of the methylene blue as an antidote was not indicated because of MetHb<30%, NAC-based antioxidant therapy was introduced to reduce MetHb levels and allow second-line therapy with primaquine for the treatment of PJP. A NAC dose of 3600 mg/day per os was established. Based on the absence of respiratory failure and the initial reduction of MetHb levels, the patient was discharged on the third day of treatment.
Afterward, the patient was followed-up as an outpatient. At each visit, the patient was evaluated both from a clinical point of view and by performing ABG. MetHb levels gradually decreased until they returned to the physiological range. The ABS performed two weeks after the patient's discharge showed a MetHb of 2% (Figure 2). Given the half-life of primaquine and its metabolites, NAC therapy lasted one week longer than the duration of primaquine therapy (for a total of 19 days) (Figure 1). No Adverse Drug Reaction (ADR) to NAC therapy has been reported.
It is well known that primaquine increases MetHb levels [8-10], through a pro-oxidant environment promoted by its metabolites [6]. Among these, 5,5-di-(8-[(4-amino-1-methylbutyl) amino]-6- methoxyquinoline) has been shown to increase MetHb levels in in vitro experiments on rat erythrocytes [13]. MetHB levels do not significantly correlate with plasma concentrations or the total administered dose of primaquine [14].
MetHB levels increased, ranging from 2.0% to 15.6%, in healthy volunteers receiving a standard dose (15 mg/die) of primaquine for 14 days and after one week of treatment with chloroquine [15]. Similarly, mild methemoglobinemia has been seen in Javanese migrants with normal G6PD activity who took 30 mg of primaquine for 20 weeks to prevent malarial infection [16].
Carmona-Fonseca, et al. [5], showed that high doses of primaquine (from 0.58 to 1.17 mg/kg/day) in individuals with normal G6PD activity do not increase methemoglobinemia by over 20%. However, symptoms associated with primaquineinduced mild methemoglobinemia (below 20%) occurred in 17% of cases.
Kedar, et al. [17], noted a more marked increase in MetHb levels (32%), accompanied by severe cyanosis, in a 17-years-old Indian boy after administration of 200 mg of primaquine for the treatment of Plasmodium vivax malarial infection. However, the patient was affected by CYB5R3 gene mutation leading to NADH-cytochrome b5 reductase decreased activity, which facilitates the MetHb increase [17]. In our case, after nine days of 30 mg/day primaquine treatment, we observed a mild increase in MetHb (9%) without other signs or symptoms (Figure 2).
We promptly decided to start NAC therapy to avoid a further increase in MetHb levels and to preserve primaquine second-line therapy for PJP. Indeed, NAC is known to reduce methemoglobin, at least in vitro experiments on blood samples given from healthy human volunteers with a mechanism not wholly understood, probably involving glutathione production (slow-acting scavenger property of NAC) and NAC-derived sulfane sulfur species (fast-acting scavenger property of NAC) [18]. After NAC administration, MetHb levels slowly returned to normal, with no further increase.
Our results showed the efficacy of NAC therapy in mildmoderate methemoglobinemia, thus avoiding primaquine discontinuation. Although the symptoms related to moderate methemoglobinemia are generally mild, in the case of primaquine-induced moderate increase (<20%) in MetHb levels, it could be advisable to start N-Acetyl Cysteine (NAC)-based antioxidant, which in our experience has proved to be effective and safe. Larger studies are needed to elucidate this important topic. However no adverse drug reaction to NAC therapy has been reported.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lorenzo L, Filippo B, Gianna C, Lorenzo C, Francesco G, Filippo L, et al (2023) Primaquine-Induced Asymptomatic Methemoglobinemia Treated with N-Acetylcysteine: A Case Report. J Clin Toxicol. 13:529.
Received: 22-Feb-2023, Manuscript No. JCT-23-21914; Editor assigned: 24-Feb-2023, Pre QC No. JCT-23-21914 (PQ); Reviewed: 10-Mar-2023, QC No. QC No. JCT-23-21914; Revised: 17-Mar-2023, Manuscript No. JCT-23-21914 (R); Published: 24-Mar-2023 , DOI: 10.35248/2161-0495.23.13.529
Copyright: © 2023 Lorenzo L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.