Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2017) Volume 5, Issue 3
Bacterial competitiveness appears to be an efficient way to fight pathogenic oral flora. This competition may be facilitated by probiotics, particularly in periodontal diseases. The aim of this study was to investigate the probiotic properties of 61 clinical isolates of oral lactobacilli. The inhibitory activity of the tested strains against periodontopathogens was assessed with the agar overlay technique. The results obtained, as well as those resulting from previous work, led us to select the nine most promising strains on which we conducted further tests, such as evaluating their coaggregation capacities with various oral species and the production of proinflammatory cytokines by PBMC. We also evaluated the safety of the probiotics by assessing their sensitivity to antibiotics. Their possible involvement in halitosis was investigated by testing their ability to produce volatile sulfur compounds. The results of the agar overlay technique showed that all the lactobacilli strains had an antibacterial activity against Tanerella forsythia, Treponema denticola, and Aggregatibacter actinomycetemcomitans. Fifty-two strains slightly inhibited the growth of P gingivalis and only two had no activity on F. nucleatum. The nine strains tested did not coaggregate with P gingivalis, T forsythia, T denticola or A actinomycetemcomitans. Three strains strongly coaggregated with F nucleatum. Only three strains produced lower IL-6 than the activator at the maximum concentration tested in this study. However, none of the 9 strains produced a greater amount of IL-8 than that obtained with 1 μg/ml of LPS. These results show us that all the strains were sensitive to the antibiotics tested, except for one strain which showed resistance to penicillin. The production of CSV by the strains remained below the threshold of halitosis. Among the 61 strains tested, 9 proved to be of interest including one that was particularly promising.
Keywords: Probiotics; Periodontal diseases; Bacteriotherapy; Lactobacillus; Microbial sensitivity test; Bacterial adhesion; Interleukin-6; Interleukin-8; Halitosis
Epidemiological studies have shown that chronic periodontitis is one of the most frequent chronic diseases [1]. This disease, which may lead to tooth loss, is largely associated with an imbalance of indigenous microflora, resulting in the overgrowth of periodontal pathogens such as Porphyromonas gingivalis, Prevotella intermedia and Aggregatibacter actinomycetemcomitans [2]. An essential aim in the treatment of periodontitis is to control the pathogenic dental biofilm and calculus by improving personal hygiene, thus reducing inflammation and pocket depths and promoting periodontal reattachment. In severe cases, antibiotic therapy may be required to facilitate healing. Among the antimicrobial and bacteriostatic agents, chlorhexidine is the gold standard for the treatment of periodontitis thanks to its broad-spectrum antibacterial activity. Unfortunately, it is not without long-term side effects, the first being the imbalance of the oral ecosystem caused mainly by its bactericidal properties, thus limiting its long-term use [3]. In order to maintain the balance of the oral cavity, bacterial competitiveness is a promising way to fight against the establishment of pathogenic flora. Such competition may be promoted by using probiotics.
In dentistry, probiotics have been used as useful adjuncts to reduce the development of caries, suppress oral Candida infections and control halitosis [4-6]. Recent publications have also demonstrated the potential benefit of probiotic administration for managing periodontal diseases, mainly as an adjuvant to conventional treatment. However, the mechanisms of the action of probiotics are still unclear and few studies have been carried out on the effect of autochthonous oral strains of Lactobacilli on species involved in oral diseases.
Since this complex ecosystem contains many strains, several of their functional features should be investigated first in vitro . In previous studies, we tested Lactobacilli strains capable of adhesion on oral surfaces and showing antibacterial activity against oral pathogens such as two cariogenic strains (Streptococcus mutans and Actinomyces viscosus ) [7,8]. However, before proposing a suitable product for the maintenance of oral health, other tests are required. The evaluation of inhibitory activity could be extended to other pathogens known to be involved in periodontitis. We thus aimed at investigating other properties of these strains such as their ability to coaggregate with various oral species, their resistance to antibiotics, their immunomodulation capacities and the extent to which they produce volatile sulfur compounds.
Bacterial strains and growth media
Sixty one clinical isolates of oral Lactobacilli previously tested for their adhesive capacity were investigated for their antibacterial properties against five periodontal pathogens. They were grown in MRS medium at 37°C, in anaerobic conditions. The five periodontal pathogens tested, Aggregatibacter actinomycetemcomitans ATCC33384, Treponema denticola ATCC35405, Tanerella forsythia ATCC43037, Fusobacterium nucleatum ATCC1095, and Porphyromonas gingivalis ATCC33277 were obtained commercially from the American Type Culture Collection (ATCC). They were all grown under anaerobic conditions (Genbox Anaer, BioMerieux, France). Fusobacterium nucleatum and Porphyromonas gingivalis were routinely grown in Wilkins and Chalgren Anaerobe medium supplemented with horse serum (Oxoid, France). Aggregatibacter actinomycetemcomitans was grown in Brain Heart Infusion (Oxoid, France) supplemented with cysteine chlorhydrate (Sigma Aldrich, France). Treponema denticola was grown in New Oral Spirochete medium modified according to the ATCC recommendations. Tanerella forsythia was grown in PY Medium with horse serum and NAM. According to their adhesive capacities and/or their antibacterial effect, 9 of the 61 strains were selected for further testing. These 9 strains have been previously identified by sequencing as Lactobacillus plantarum, Lactobacillus rhamnosus (6 strains), Lactobacillus gasseri and Lactobacillus kefiri .
Antibacterial activity
The inhibitory activity of the tested strains was investigated with the agar overlay technique described by Fleming [9]. Briefly, the surface of MRS agar (Fischer Scientific, France) was spot-inoculated with 2 mL of an overnight culture of the tested Lactobacilli , previously adjusted to OD of 1.0 ± 0.02 at 550 nm (three spots per dish). To allow colonies to develop, agar plates were incubated for 1 day at 37°C in anaerobic conditions (Genbox Anaer, BioMerieux, France). They were then overlaid with 7 mL of Brain Heart Infusion (Oxoid, France) soft agar (0.75% agar), supplemented with cysteine chlorhydrate (1 g/l), hemin (10 mg/l), menadione (1 mg/l), yeast extract (5 g/l) and tryptone (3 g/l), which had been seeded with 0.1 mL of the bacteria culture to be tested. A clear zone around the Lactobacilli colonies was recorded as positive inhibition.
Coaggregation
Coaggregation was measured as previously described by Nagaoka [10]. Bacterial cells were harvested by centrifugation at 3000 g for 20 min and washed twice in physiological saline. Each pellet was resuspended in a coaggregation buffer (CB) to yield an OD600 nm of 1.0. CB comprised 0.1 mM CaCl2, 0.1 mM MgCl2, 0.15 M NaCl, and 3.1 mM NaN3 dissolved in 1 mM Tris, and the pH was adjusted to 8.0. Equal volumes (0.5 mL) of the cell suspensions of a Lactobacillus strain and an oral pathogen strain were mixed in a cuvette, and the OD600 nm was measured immediately (time-zero value). After incubation at room temperature for 90 min, the OD600 nm was measured again (sample value). The percentage (%) of coaggregation was calculated as follows: T0 value-Sample value/T0 value × 100. When the coaggregation percentage was under 10%, it was considered that there was no coaggregation. A coaggregation percentage between 10% and 30% corresponded to low aggregation, between 30% and 40% to mild coaggregation and over 40% to strong coaggregation.
Antibiotic resistance
Antibiotics resistance was tested with the antibiogram dilution method according to the EU-Prosafe project [11]. The Lactobacillus strains were tested for their antibiotic susceptibilities by broth microdilution, using the LAB susceptibility test medium (LSM) described by Klare et al. [12]. Overnight cultures of the strains on MRS agar were resuspended in physiological saline. The optical density at 625 nm (OD625 nm) was adjusted to obtain a value ˜ 0.1 (corresponding to about 0.5 Mcfarland) according to the standards recommended by the CLSI (Clinical and Laboratory Standard Institute, USA) for carrying out antibiogram tests. One µl of the Lactobacillus suspensions was inoculated in each dilution of each antibiotic to be tested (final volume of each well of the microplate=50 µL). Immediate reading after inoculation (T0) and 24-hour reading (T24) were performed using the PowerWave microplate reader (Biotek). The following antimicrobials were tested in the concentration ranges (mg/L) given between brackets: Penicillin (0.032-64), Ampicillin (0.032-64), Gentamycin (1-2048), Streptomycin (2-4096), Vancomycin (0.125-256), Erythromycin (0.016-32), Clindamycin (0.032-32), Oxytetracycline (0.063-128), Chloramphenicol (0.125-256).
Interpretation of susceptibility status was based on the most recent FEEDAP document [13] and on the reference values given by Klare et al. [14].
Immunogenic properties
Peripheral blood mononuclear cells: PBMC were obtained from healthy volunteers blood. Sampling was performed on the day of the experiment; blood was diluted part to part in RPMI 1640 without FBS (fetal bovine serum) (Lonza, Basel, Switzerland) and 10 mL of diluted blood were deposited on 5 mL MSL. After centrifugation at 2000 tr/min (30 min 18°C), the cell ring was sampled and centrifuged (1800 tr/min 18 min at 18°C). The cells were suspended in RPMI 1640 with 5% FBS at a final concentration of 1.106 cells/mL. Five hundred µl of the suspension were then deposited in each well of a 24-well microplate.
Probiotic strains and growth conditions: Before the experiment, overnight cultures of the Lactobacilli were prepared on MRS agar (AES, Rennes, France) at 37°C in an atmosphere of 5% CO2. A suspension was prepared in MRS broth on the day before the experiment (transmission 50%, 550 nm) and was then diluted 1/10 and incubated overnight. On the day of the experiment, the suspension was centrifuged (3600 tr/min, 10 min) and adjusted at 9.108 CFU/mL in PBS. Dilutions were performed in RPMI with 5% FBS to obtain the final bacteria-to-cell ratio of 4.5/1, 2/1, 0.5/1, 0.25/1, 0.125/1.
Assay conditions
Microplates containing PBMC and bacteria in a different ratio were incubated for 24 h at 37°C under 5% CO2. At the end of the incubation, the supernatants of each well were collected by aspiration and cell debris were removed by centrifugation (1600 tr/min, 10 min at 4°C) and stored at -20°C. A negative control was obtained from cells without bacteria suspension (addition of 500 µl RPMI with 5% FBS). A positive control was obtained by addition of LPS (1 µg/mL) to the cells.
IL-6 and IL-8 ELISA quantification
The quantification of secreted IL-6 and IL-8 was performed by ELISA (KIT R&D Systems). After OD reading (450 nm), the percentage of variation in IL-6 or IL-8 secretion activation was calculated as follows:
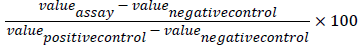
Production of Volatile Sulfur Compounds (VSC)
VSC production was analyzed by gas chromatography. Overnight cultures of each strain tested were inoculated into four different media: MRS broth, MRS broth supplemented with methionine (0.1 mg/mL), MRS broth supplemented with cysteine (0.1 mg/mL), MRS broth supplemented with methionine (0.1 mg/mL) and cysteine (0.1 mg/ mL).
Determination of low boiling point sulfur compounds, hydrogen sulfide, methane thiol (MetSH), ethane thiol (EtSH), dimethyl sulfide (DMS), carbon disulfide and dimethyl disulfide (DMDS) was carried out from a starting sample of 10 ml (filtered medium or resting cell suspension) transferred into a 50 ml hermetically closed vial after addition of 10 µl of 318 mg/L thiophene (internal standard). The sample was homogenized for 2 min and maintained for 20 min at room temperature to allow all tested sulfur compounds to diffuse similarly in both the liquid and gas phases. One milliliter of gas was injected into the gas chromatograph (HP 5890, Agilent Tech., France) equipped with an HP 5 (30 m × 0.53 mm, 5 µm, Agilent Tech., Montluçon, France) column and coupled to a flame photometric detector (FPD).
Antibacterial activity
The antibacterial activities of 61 salivary strains of Lactobacilli against five periodontopathogenic bacteria (P. gingivalis , F. nucleatum , T. forsythia , T. denticola , A. actinomycetemcomitans) were evaluated. The results of the agar overlay technique show that all the strains had an antibacterial activity against T. forsythia , T. denticola , A. actinomycetemcomitans over a 96 h period. Fifty-two strains slightly inhibited the growth of P. gingivalis and only two had no activity on F. nucleatum . The inhibitory zones for F. nucleatum ranged from 9 mm to 40 mm, for T. forsythia from 14 mm to 40 mm, for A. actinomycetemcomitans from 9 mm to 40 mm, and for T. denticola from 15 mm to 40 mm. For P. gingivalis , they ranged from 7 mm to 18 mm (Figure 1).
Co-aggregation
The 9 strains tested were unable to co-aggregate with P. gingivalis , T. forsythia , T. denticola or A. actinomycetemcomitans . Two strains did not coaggregate with F. nucleatum . Four showed low coaggregation. Only 3 strains co-aggregated strongly with F. nucleatum (Table 1).
| 22B | 31A | 33A | 34A | 52B | 57A1 | BAP3 | BMS2 | CJS1 | |
|---|---|---|---|---|---|---|---|---|---|
| P gingivalis | 2.89 | 0.32 | 0.32 | 0 | 0.33 | 1.31 | 4.53 | 2.59 | 3.56 |
| Fnucleatum | 19.68 | 18.87 | 20.37 | 17.29 | 4.02 | 5.86 | 48 | 60.07 | 40.06 |
| A actinomycetemcommitans | 0 | 2.36 | 0.66 | 0.66 | 3.6 | 2.31 | 2.41 | 0.34 | 0.67 |
| Tdenticola | 0.63 | 0 | 0.62 | 0.31 | 0 | 0 | 0.66 | 0.65 | 0.96 |
| T forsythia | 0.93 | 1.91 | 0.32 | 0.65 | 1.27 | 0.98 | 1.95 | 3.22 | 0.64 |
Table 1: Percentage of co-aggregation.
Antibiotics resistance
Table 2 summarizes the concentration at which the growth of each strain was inhibited for each given antibiotic. All the strains were sensitive to the antibiotics tested, except for strain 22 B which showed resistance to penicillin (Table 2).
| Ampi | Chlor | Erythro | Oxyt | Clind | Genta | Peni | Strepto | Vanco | |
|---|---|---|---|---|---|---|---|---|---|
| L gasseri | 0.5 | 0.5 | 0.016 | 2 | 0.016 | 0.25 | 0.5 | 1 | 1 |
| 22B | |||||||||
| L rhamnosus31A | 1 | 0.063 | 0.016 | 0.5 | 0.016 | 0.125 | 0.063 | 4 | 4 |
| L rhamnosus 33A | 1 | 0.5 | 0.016 | 0.5 | 0.016 | 0.125 | 0.063 | 4 | 4 |
| L rhamnosus34A | 2 | 0.5 | 0.016 | 0.5 | 0.016 | 0.125 | 0.063 | 4 | 4 |
| L rhamnosus52B | 1 | 1 | 0.016 | 0.5 | 0.016 | 0.125 | 0.063 | 4 | 4 |
| L rhamnosus 57A1 | 2 | 0.25 | 0.016 | 0.5 | 0.016 | 0.125 | 0.063 | 4 | 4 |
| L fermentum | 0.5 | 1 | 0.016 | 1 | 0.016 | 0.032 | 0.063 | 8 | 2 |
| BAP3 | |||||||||
| L plantarum | 1 | 0.5 | 0.016 | 16 | 0.016 | 0.063 | 0.063 | 1 | 1 |
| BMS2 | |||||||||
| L kefiri CJS1 | 0.125 | 0.5 | 0.016 | 1 | 0.016 | 0.063 | 0.063 | 4 | 0.25 |
Table 2: Minimum Inhibitory Concentrations (mg/L).
Immunogenic properties
The in vitro treatment of PBMCs by the 9 lactobacillus strains for 24 hours resulted in an increased release of pro-inflammatory cytokines IL-6 and IL-8. This response was dose-dependent for all strains tested. Only three strains (31A, 33A and BMS2) produced less IL-6 (by PBMC) than the activator (LPS at 1 µg/mL) for the maximum concentration tested in this study (4.5 bacteria per cell). However, for IL-8 production by PBMCs in the presence of Lactobacilli , none of the 9 strains produced (at the tested concentrations) a greater amount of IL-8 than that obtained with 1 µg/ml of LPS.
| Strain | Bact/PBMC ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.5 : 1 | 02:01 | 01:01 | 0.5 : 1 | 0.25 : 1 | 0.125 : 1 | |||||||
| IL-6 | IL-8 | IL-6 | IL-8 | IL-6 | IL-8 | IL-6 | IL-8 | IL-6 | IL-8 | IL-6 | IL-8 | |
| 22B | 324 | 443 | 269 | 289 | 206 | 212 | 144 | 103 | 85 | 55 | 49 | 32 |
| 31A | 244 | 302 | 160 | 154 | 122 | 86 | 76 | 43 | 61 | 30 | 37 | 23 |
| 33A | 273 | 298 | 207 | 172 | 154 | 102 | 107 | 59 | 47 | 28 | 36 | 22 |
| 34A | 302 | 366 | 202 | 222 | 163 | 155 | 80 | 53 | 60 | 33 | 25 | 25 |
| 52B | 342 | 406 | 397 | 285 | 209 | 160 | 118 | 86 | 77 | 42 | 56 | 34 |
| 57A1 | 343 | 378 | 220 | 189 | 155 | 120 | 104 | 56 | 58 | 34 | 23 | 24 |
| BAP3 | 241 | 340 | 214 | 276 | 183 | 266 | 125 | 256 | 114 | 163 | 94 | 85 |
| BMS2 | 181 | 328 | 143 | 284 | 129 | 267 | 112 | 185 | 152 | 120 | 98 | 63 |
| CJS1 | 345 | 357 | 280 | 341 | 206 | 201 | 116 | 81 | 77 | 39 | 33 | 26 |
Table 3: Effect of Lactobacillus strains on production of IL-6 and IL-8 by PBMC.
Production of volatile sulfur compounds
No ethanethiol, dimethyl sulfur or carbon disulfide was detected in our strains incubated in cysteine and/or methionine-supplemented medium. Hydrogen sulfide was detected in all strains, mostly at levels ranging from 4 µg/l to 10 µg/l. Only one strain produced a high level of H2S (162.5 µg/l). All the strains tested produced methanethiol (18.8 µg/l to 84 µg/l) and dimethyl disulfide (11.6 µg/l to 104.2 µg/l).
The present study investigated the probiotic properties of clinical isolates of lactobacillus strains that could be used for preventing or treating periodontitis. Our previous study showed that some strains may be of interest for probiotic use in the oral cavity. It was therefore decided to continue investigations on these strains in order to reveal other interesting properties or to rule out those unsuitable for use as probiotics [7,8].
The results indicate that among the 61 tested strains, 9 showed promising antibacterial activity against bacteria involved in periodontitis. Other authors have obtained similar results on growth inhibition of certain periodontopathogens by Lactobacilli but the use of different methods makes comparison difficult [15,16].
The ability to aggregate is considered a beneficial property for probiotic strains [17-20]. Although adhesion tests had already been carried out, we wanted to test coaggregation because it is involved at two levels. First, it has an antibacterial action, as the formation of complexes with pathogens assists their elimination by salivary clearance. Moreover, the proximity of the bacteria within these complexes promotes the action of the antibacterial molecules. Second, this phenomenon of coaggregation intervenes in the formation of dental biofilm. Our Lactobacillus strains did not possess strong aggregation capacities with the pathogens tested. In fact, the values were low, except for F. nucleatum , and only for certain strains. This is mainly due to the properties of F. nucleatum , which acts as a bridge between several species in the constitution of the dental biofilm. Indeed, in the absence of F. nucleatum , many secondary colonizers cannot become part of the dental plaque community [21]. These results are in accordance with those obtained by Jang et al. on other probiotic species [22].
It is now well known that most Lactobacilli are susceptible to various antibiotics, even when they are involved in diseases [23]. It is therefore essential to verify the safety of the probiotics that may be used in the oral cavity by checking for the absence of antibiotic resistance [14]. Nine antibiotics were tested. All the strains were sensitive to the antibiotics tested and showed antibiotic susceptibility profiles with values below the thresholds set by the European Food and Safety Authority (EFSA), with the exception of L. gasseri , which appeared to have a slight resistance to penicillin compared to the usual inhibition values. The strains are therefore usable as probiotics, even if one of the strains seems to have less potential. Similar results have been obtained by Hütt et al. [24]. However, they showed in a more recent study that Lactobacilli of vaginal origin could have multiple resistances. They concluded that multiple antibiotic resistances may be speciesdependent [25].
Many studies have shown that an in vitro PBMC model is a good screening tool to identify the characteristic traits of probiotic strains [26-28]. Therefore, our experiment was carried out on peripheral blood mononuclear cells (PBMCs) and the determination of the IL-6 and IL-8 proinflammatory cytokines showed the response of the PBMCs to stimulation by the Lactobacilli . The results showed four bacteria of interest that led to a lower release of IL-6 than that of the activator. All strains resulted in a release of IL-8 lower than that of the activator.
This result is promising because the ability of probiotics to reduce in vitro IL-8 levels is well documented and serves as one of the basic criteria for selecting probiotic bacteria with this potential [29]. To our knowledge, only few studies are available on the modulation of IL-6 production by probiotics. However, Kobayashi et al. showed that IL-6 mRNA and protein levels following P. gingivalis infection were significantly reduced by the oral administration of the probiotic strain L. gasseri before infection. They suggested that this strain had a preventive effect against P. gingivalis -induced experimental periodontitis by regulating inflammatory reactions [30].
The possible implication in halitosis of the strains tested was investigated by their ability to produce volatile sulfur compounds (VSC). Most studies to date have focused on the role of probiotics in inhibiting the production of VSC. Thanks to our results on the inhibition of potentially VSC-producing bacteria, we suggest that our strains may have an inhibitory effect on this production, as Masdea et al. have shown on gram-positive bacteria involved in halitosis [31] and Lee and Baeck on Porphyromonas gingivalis [32]. As part of our safety tests, we also studied the production of VSC by our strains. Fusobacteria are among the most prolific VSC-producing species found in the oral biofilm, as suggested by data in various studies [33-35]. We found that our strains of Lactobacilli were able to coaggregate with Fusobacterium nucleatum in planktonic form. This suggests that they could be closely linked to the oral biofilm of individuals with halitosis. Our strains produced fewer VSC than S. moorei. However, Stephen et al. have shown that S. moorei is a moderate producer of H2S compared with other oral bacteria such as F. nucleatum and P. melaninogenica [33]. We therefore think that our strains do not play a role in halitosis.
Further studies are necessary to identify a probiotic strain that shows neither harmfulness nor side-effects in animal models and in human trials. Clinical trials are indeed essential to demonstrate the action of these probiotic candidates on periodontal health. It also seems useful to examine which product form and dosage would be sufficient to optimize their retention in the oral cavity.
This work was supported by Aquitaine Science Transfert. Authors report no conflict of interest.