
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 4
Sepsis is one of the commonest problems associated with the admission of critically ill patients, especially HIV/AIDS patients at the Critical Care Units of Hospitals. Diagnosis and prognosis of sepsis in the clinical setting is a challenge since there is no diagnostic tool for measuring the severity and course of sepsis. Therefore, this study was designed to assess the use of Procalcitonin as a surrogate marker of sepsis in a case-control study of both septic and non-septic HIV/AIDS and also for the determination of its cut-off threshold for sepsis.
Using a hospital-based case–control study design, 100 HIV/AIDS patients comprising of 66 septic cases and 34 nonseptic were recruited from the Emergency Department of Komfo Anokye Teaching Hospital (KATH), Ghana. Sepsis was defined using the Systemic inflammatory response syndrome (SIRS) criteria. Serum Procalcitonin (PCT) and Creactive protein (CRP) levels were measured by standard ELISA principle.
Prevalence of the overall sepsis, was assessed at 60. 5% among patients who were on Anti-retroviral therapy (ART), 66. 7% in females and 33. 3% in males. Viral suppression was 71% in septic patients. The overall mortality rate recorded was 89. 4%. ART however, did not confer protection from death, as 56. 5% of cases on ART died.
Only 22. 7% of the septic patients produced positive blood cultures. PCT was significantly higher in septic patients than in non-septic patients (p=0. 000) and also higher in patients with positive blood cultures as compared with negative blood cultures (p=0. 001). PCT levels were significantly high in patients who died as compared to those who survived (p=0. 000). The AUC of PCT and CRP were 0. 968 and 0. 726 at cut-offs of 0. 496 ng/ml and 39. 281 mg/L respectively for diagnosing sepsis in HIV/AIDS patients. The study clearly shows that PCT is a better surrogate marker for diagnosing sepsis in people living with HIV/AIDS as compared to CRP.
Procalcitonin; C-reactive protein; Viral load; White blood cell; Anti-retroviral; Systemic inflammatory response syndrome; Intensive care unit
Sepsis is an old disease in medicine which has been recognized since 1, 000 BC in several different forms and its detection and treatment has gained profound interest in researchers for about 3, 000 years [1,2]. It is defined as the host’s immune response to injury and/or infectious stimuli in the presence of a known infection. Systemic inflammatory response Syndrome criteria has been employed in defining sepsis [3]. Systemic inflammatory response Syndrome (SIRS) was diagnosed when a patient meets any two of the following criteria: fever or hypothermia (body temperature ≥ 38°C or <36°C), tachycardia (heart rate >90/min), tachypnoea (respiration>20/min or PaCO2<32 mmHg), and leukocytosis or leukopaenia (white blood cell (WBC) count >12. 0×109/L or <4.0×109/ L) neutrophilia (neutrophil count >10% immature (band) forms. Sepsis was described as systemic inflammatory response (thus once a patient has any two of the above symptoms) to an infection.
Patients infected with Human immunodeficiency virus/ Acquired Immune Virus (HIV/AIDS) are at higher risk of developing sepsis as compared with patients without HIV infections since their reduced innate immune response increases their susceptibility to sepsis infection [4,5]. Epidemiological studies show that about 85% of septic patients admitted at hospitals are HIV positive [6,7]. The occurrence of sepsis and severe sepsis is a key factor of outcomes in critically ill HIV/ AIDS patients, which influences short- and long-term existence [8]. A study conducted recorded a mortality rate of 50% in HIV/AIDS patients despite of Antiretroviral therapy (ART) use.
Diagnosis of bloodstream infection remains a challenge to Clinicians and Physicians since the symptoms of sepsis are nonspecific [9]. Blood culture which is the diagnostic tool for microbial infections delays in obtaining results. This delay in treatment and worsen the outcome and response time of antimicrobial treatment [4]. PCT has been employed extensively as a diagnostic marker for the early detection and monitoring of severe bacterial infections and sepsis [10]. The concentration of PCT in a healthy individual ranges from undetectable to less than 0. 1 ng/ml. Sepsis is usually suspected when the concentration is 0. 5 ng/ml and can be as high as within the range of 100 ng/ml to 1000 ng/ml in some patients [11]. In conjunction with other clinical data, PCT indicates whether antibiotic therapy appears necessary since the course of PCT also reflects the efficacy of antibiotic therapy and thus indicates when it should be terminated. This substantially reduces the cost, high mortality risk and complications associated with sepsis in HIV/AIDS [12]. Therefore, this novel study was designed to assess the use of Procalcitonin as a surrogate marker for sepsis in a case-control study among septic and non-septic HIV/AIDS patients and also to determine its threshold cut-off.
Study design and settings
The study was a hospital-based case-control which was conducted from February 2017 to March 2018 at the Accident and Emergency department of the Komfo Anokye Teaching Hospital (KATH) in the Ashanti Region of Ghana. It is the main referral Centre that delivers health services to more than five regions in Ghana.
Study population
Patients who presented to the Accident and Emergency Department of Komfo Anokye Teaching Hospital who were HIV positive (patient reported or documented in patient chart) was approached to be consented for the study. Convenience sampling technique was used to recruit 100 patients (comprising of 66 cases and 34 controls). Patients who met the following SIRS criteria were considered to have sepsis and patients who did not meet the SIRS criteria were considered as not having sepsis and thus used as controls. SIRS was present when a patient met any two or more of the following criteria; fever or hypothermia ( ≥ 38°C or<36°C), tachycardia (heart rate>90/ min), tachypnoea (respiration>20/min or PaCO2<32 mmHg), and leukocytosis or leukopaenia (white blood cell (WBC) count>12. 0 × 109/L or<4. 0 × 109/L) neutrophilia (neutrophil count>10% immature (band) forms [3]. Body temperature, heart rate and respiratory rate were assessed and recorded by the infectious disease specialist Physician on team where as white blood cell count was obtained from the laboratory results of patients complete cell count using blood samples.
Inclusion and exclusion criteria
Confirmed HIV seropositive patients who were 18 years and above and voluntarily consent to take part in the study were recruited on day one of their admission to hospital. All patients who had renal failure and/or were in an immediate postoperative period were excluded from the study since they presented with post-septic elevated levels of PCT levels.
Questionnaire administration and data collection
Structured questionnaires were used to obtain sociodemographics such as occupation, age and marital status of each participant. Furthermore, various identified risk factors such as chronic disease, family history of chronic disease, previous admission at hospital, length of hospital stay during previous admissions, HIV/AIDS infection duration, Antiretroviral therapy (ART) as well as history of co-infections were obtained using questionnaires.
Sample size
The estimated minimum sample size was calculated to be 100 using the Kish-Leslie formula; n=t2 × p (1-p)/m2; where n=sample size, p is the prevalence sepsis in HIV/AIDS, m is the margin of error [13].
Clinical and laboratory data
Clinical and laboratory data including body temperature, respiratory rate, height, weight were recorded. Complete blood count was performed using Sysmex KX4000i haematology analyser (Sysmex Corporation, Kobe, Japan). PCT was analysed usingprinciple by Thermo Scientific Co. Ltd and C-reactive protein (CRP) was analysed using the principle by Cayman Chemical Co. Ltd. The viral load count was done using COBAS ® AmpliPrep/COBAS ® TaqMan ® HIV-1 Test. Clinical assessments of all patients including reviews of patients’ folders to assess the need for special care was carried out by an infectious disease specialist Physician on the team. Blood cultures were done using the manual method by following the procedures laid out by the various agar manufacturers.
Data analysis
Normality of all continuous variables was tested. Continuous variables were stated as mean ± Standard deviation (SD), and categorical variables were stated as frequency (n) and percentages (%). Comparisons ofProcalcitonin concentration of the septic group against the non-septic group were done using One Way ANOVA, chi (χ2) tests, or Fisher exact tests where appropriate. A level of p<0. 05 was described as statistically significant for all analysis. IBM Statistical Package for the Social Sciences (SPSS) version 20. 00 and GraphPad Prism version 6. 00 were used for statistical analysis where appropriate (SPSS Inc., Chicago, USA ; GraphPad software, San Diego California USA).
Socio-demographic characteristics of participants
This study recruited 100 HIV/AIDS patients. Out of the 100 HIV/AIDS positive participants recruited, 66 were cases who met the SIRS criteria of defining sepsis and 34 were controls who did not meet the SIRS criteria of defining sepsis. There was no significant difference (p=0. 393) in ages. Higher proportions of the participants were within the age range of 31-40 years. Majority of the cases were females (66. 7%). There were no significant differences in the socio-demographic characteristics of cases and controls, showed in Table 1.
| Parameter | Cases | Controls | p value |
|---|---|---|---|
| (N=66) | (N=34) | ||
| Age (years) | 41. 5 ± 11. 2 | 39. 6 ± 8. 9 | 0. 393 |
| Age group (years) | 0. 916 | ||
| 21-30 | 9 (13. 6) | 5 (14. 7) | |
| 31-40 | 28 (42. 4) | 15 (44. 1) | |
| 41-50 | 18 (27. 3) | 11 (32. 4) | |
| 51-60 | 6 (9. 1) | 2 (5. 9) | |
| 61-70 | 4 (6. 1) | 1 (2. 9) | |
| 71-80 | 1 (1. 5) | 0 (0. 0) | |
| Gender | 0. 18 | ||
| Male | 22 (33. 3) | 16 (47. 1) | |
| Female | 44 (66. 7) | 18 (52. 9) | |
| MS | 0. 978 | ||
| Married | 30 (45. 5) | 17 (50. 0) | |
| Single | 21 (31. 8) | 10 (29. 4) | |
| Divorced | 9 (13. 6) | 5 (14. 7) | |
| Co-habitation | 3 (4. 5) | 1 (2. 9) | |
| Widowed | 3 (4. 5) | 1 (2. 9) | |
| Occupation | 0. 859 | ||
| Unemployed | 13 (19. 7) | 6 (17. 6) | |
| Formal | 7 (10. 6) | 2 (5. 9) | |
| Informal | 44 (66. 7) | 25 (73. 5) | |
| Student | 2 (3. 0) | 1 (2. 9) | |
Table 1: Association of socio-demographic characteristics of participants.
Physiological and biochemical characteristics of participants
The physiological and biochemical characteristics of the participants were determined from vital signs and blood samples and there was statistically significant difference in the means of heart rate (p=0. 001), temperature (p=0. 012), respiratory rate (p<0. 001) and procalcitonin levels (p=0. 000) between the cases and controls. There was no statistically significant difference in the white blood count, platelets count, systolic and diastolic blood pressures between cases and controls. However, the means of the platelet count in cases was higher (210. 70 ± 127. 41) than the means in the control group (239. 88 ± 123. 25) (Table 2).
| Parameter | Cases | Controls | p value | |
|---|---|---|---|---|
| (N=66) | (N=34) | |||
| WBC (× 109/L) | 8. 60 ± 5. 20 | 7. 04 ± 3. 52 | 0. 119 | |
| PLT (× 109/L) | 210. 70 ± 127. 41 | 239. 88 ± 123. 25 | 0. 275 | |
| Heart Rate (bpm) | 111. 27 ± 24. 08 | 93. 97 ± 22. 74 | 0. 001* | |
| Temperature (°C) | 37. 45 ± 1. 36 | 36. 82 ± 0. 62 | 0. 012* | |
| Respiratory Rate (cpm) | 25. 35 ± 7. 00 | 20. 29 ± 1. 96 | 0. 000* | |
| SBP (mmHg) | 117. 73 ± 24. 36 | 111. 12 ± 19. 73 | 0. 175 | |
| DBP (mmHg) | 77. 35 ± 18. 48 | 74. 24 ± 15. 26 | 0. 401 | |
| ART use in patients | 0. 366 | |||
| Yes | 23(60. 5) | 15(39. 5) | ||
| No | 43(69. 4) | 19(30. 6) | ||
| Viral load/cp/ml | 3. 80 × 105 ± 13. 76 × 105 | 3. 34 × 105 ± 14. 97 × 105 | 0. 878 | |
| Viral suppression | 0. 482 | |||
| <1000 cp/ml | 22(71. 0) | 9(29. 0) | ||
| >1000 cp/ml | 44(63. 8) | 25(36. 2) | ||
| Procalcitonin/ng/ml | 0. 6111 ± 0. 194 | 0. 386 ± 0. 038 | 0. 000* | |
| C-reactive Protein/mg/ml | 38. 187 ± 13. 345 | 35. 777 ± 11. 765 | 0. 977 | |
| Outcome | 0. 000* | |||
| Discharged | 24(45. 3) | 29(54. 7) | ||
| In hospital mortality | 42(89. 4) | 5(10. 6) | ||
Table 2: Physiological and biochemical characteristics of participants.
Microbiological cultures
Microbiological cultures done on the blood samples of the participants showed fifteen (15) bacteria growth (22. 7%) in the 100 participants, recording no growth in controls. Organisms isolated from the 15 positive microbial cultures included Staphylococcus aureus, Streptococcus species, Streptococcus pneumoniae, Klebsiella species, Bacillus species, Haemophilus influenza, Escherichia coli, and fungi representing 3. 0%, 3. 0%, 3. 0%, 3. 0%. 1. 5%, 1. 5%, 1. 5%, and 6. 1% of the positive cultures respectively (Figure 1).
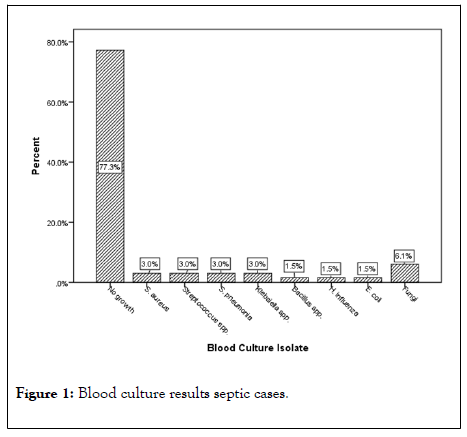
Figure 1: Blood culture results septic cases.
Procalcitonin concentrations
The Procalcitonin level was analyzed to identify any relationship between PCT levels and blood culture results. There was a significant difference (p=0. 001) in the procalcitonin level between negative and positive cultures. Procalcitonin was significantly high in patients with positive cultures, which confirms bacterial sepsis. This is consistent with clinical implications of increased procalcitonin levels and the risk of sepsis infection. Procalcitonin was significantly high in septic patients who died during the in-hospital stay period than in patients who survived (p value of 0. 000) (Table 3).
| Parameter | Procalcitonin/ng/ml | p value |
|---|---|---|
| Blood culture | 0. 001* | |
| Negative | 0. 571 ± 0. 184 | |
| Positive | 0. 749 ± 0. 164 | |
| ART treatment | 0. 158 | |
| ART users | 0. 565 ± 0. 232 | |
| No ART use | 0. 636 ± 0. 168 | |
| Outcome | 0. 000* | |
| Discharged | 0. 478 ± 0. 225 | |
| Died | 0. 687 ± 0. 123 | |
Table 3: Association of Procalcitonin with clinicaloutcomes of septic patients.
ROC curve characteristics of Procalcitonin and Creactive protein
Table 4 shows the diagnostic yields of Procalcitonin and Creactive protein. Using a cut-off value of 0. 4960 ng/ml Procalcitonin had a sensitivity of 96. 1% and specificity of 92. 9% with 100% negative predictive value and positive predictive value 98. 1% and p value of 0. 000. With C-reactive protein, a cut-off value of 39. 281 mg/ml/ml yielded a sensitivity of 56. 5. 1% and specificity of 100% for the detection of sepsis with a positive predictive value of 100% and negative predictive value of 71%.
| Markers | Cutoff | Sensitivity | Specificity | PPV | NPV | AUC (p value) |
|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | |||||
| Procalcitonin/ng/ml | 0. 496 | 96. 10% | 92. 90% | 98. 10% | 100% | 0. 968(p=0. 000) |
| C-reactive protein /mg/ml | 39. 281 | 56. 50% | 100% | 100% | 71% | 0. 726(p=0. 004) |
Table 4: ROC curve characteristics of Procalcitonin and C-reactive protein.
Figure 2 shows ROC curve which depicts the accuracy of Procalcitonin in diagnosing sepsis in HIV/AIDS patients. The AUCs for Procalcitonin was 0. 968.
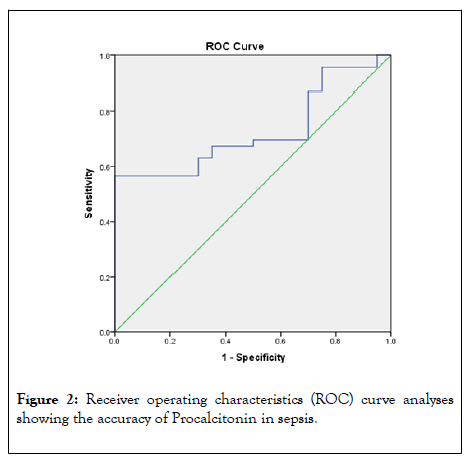
Figure 2: Receiver operating characteristics (ROC) curve analyses showing the accuracy of Procalcitonin in sepsis.
Figure 3 shows ROC curve which depicts the accuracy of Creactive protein in diagnosing sepsis in HIV/AIDS patients. The AUCs for C-reactive protein was 0. 726.
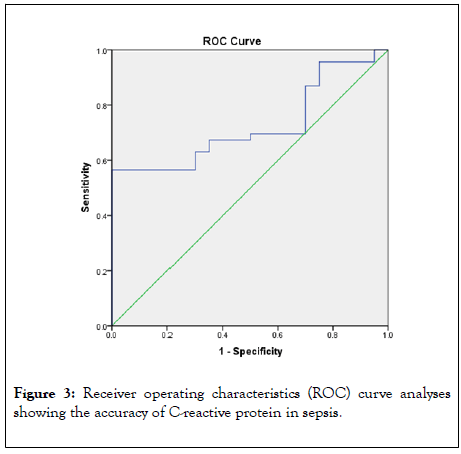
Figure 3: Receiver operating characteristics (ROC) curve analyses showing the accuracy of C-reactive protein in sepsis.
Sepsis is one of the common problems associated with the admission of critically ill patients especially HIVV/AIDS patients at the critical care units [14]. Diagnosis and prognosis of sepsis are mostly based on positive blood cultures and white blood cell count. However, positive blood culture delays in obtaining results, as white blood cell count remains non-specific leading to delay in diagnosis and treatment, consequently resulting in poor outcomes [15].
This study demonstrated no significant association of patient’s age, gender, marital status, occupation, residence and income with sepsis. Although no statistically significance difference was observed between the cases and controls, septic patients were older (41. 5 ± 11. 2 vs 39. 6 ± 8. 9).
There was statistically significant difference between cases and controls with respect to heart rate (p=0. 001), respiratory rate (p=0. 000) and body temperature (0. 012) which are clinically the main symptoms of suspecting sepsis that was used in this study [3]. Majority of the septic patients in this study were between the ages of 31-40.
ART did not confer any protection from sepsis (p=0. 366) as 60. 5% of the patients on ART developed sepsis infection after therapy. This was similar to the findings of Amancio, who concluded that 57% of HIV/AIDS patients on ART developed sepsis [8]. The risk factors in ART treatment in relation to sepsis infection could be due to viral resistance, inappropriate ART combinations, host genetic factors or patients defaulting in taking treatments [5]. Patients who were not on ART however were at higher risk (69. 4%) of developing sepsis with (80%) of the positive blood cultures coming from this population. High viral load is thought to be associated with increasing risk of sepsis. In this study an average viral load of 3. 80 × 105 ± 13. 76 × 105 was recorded in the cases.
The mortality rate of sepsis observed in this study was 89. 4%. This is the highest mortality rate recorded so far and is in line with the findings of Japiassu et al. (2010) who reported the mortality rate among septic HIV/AIDS patients to be 66% in a prospective cohort study [7]. Joao et al, 2013 did a prospective observational study among 58 patients of which 36 were HIV/ AIDS patients admitted at the intensive care unit. They recorded 57% mortality rate during in hospital stay [5]. Jacob and 382 patients admitted in Uganda with sepsis of which 85% of the patients were HIV positive and recorded a mortality rate of 64% among the septic patients [16]. The direct comparison of mortality rates between populations and hospital-based studies is challenging owing to the difference in sample size and study design. However, it is observed that mortality rate of sepsis is high among HIV/AIDS patients [4]. High mortality rate in septic HIV/AIDS patients are mostly recorded to have been caused by the underlying chronic disease, however, sepsis can be the major factor leading to increasing death and therefore much attention must be given to its treatment.
This study recorded 22. 7% positive blood cultures. This finding was consistent with the findings of Junyan and Shevin. In the findings of Junyan et al. (2013), positive blood cultures were confirmed in 17. 79% of the patients [17]. Another work done recorded positive blood cultures in 30% of cases and the causative organisms identified Escherichia coli (22%), Staphylococcus aureus (15%), and Streptococcus Pneumonia (8%-12%) [18]. Organisms isolated from this study were Staphylococcus aureus, and Streptococcus Pneumonia, Escherichia coli, Streptococcus species, Klebsiella species, Bacillus species, Haemophilus influenza and fungi. In a study by Japiassu et al. (2010), a high value of 43% positive blood cultures was recorded as Rodrigo et al. (2013) reported 33% bacteremia in their study. This is consistent with the results obtained in this work though positive cultures were higher in their work. The differences in the bacteremia among the various studies could be associated with the microbial methods, the health care center and expertise of researchers. This research was conducted in a referral hospital. Patients being referred to this hospital might have been exposed to antimicrobials prior to referral, which resulted in no microbial growth.
The mean Procalcitonin concentration in this study was 0. 6111 ng/ml in the case group which was statistically significant (p=0. 000) as compared to that of the control group which was 0. 386 ng/ml. Clinically, PCT levels are low in healthy individuals, ranging from undetectable to 0. 1 ng/ml but increase to about 100 folds in sepsis infection [11]. Sepsis is always suspected at a PCT concentration of 0. 5 ng/ml [11]. The mean concentration of Procalcitonin observed in the cases group of this study correlate with the clinical PCT levels suggestive of bacterial infections. The mean concentration of CRP was 38. 187 mg/L in cases and 35. 777 mg/ml in controls, there was statistically no significant difference between cases and controls with respect to sepsis infection. This is supported by findings a prospective study, which reported CRP mean concentration of 37. 0 mg/L among the 52 AIDS patients enrolled in the study [19]. High or increasing levels of PCT are always associated with bad outcome whereas low or decreasing levels are associated with better outcome. The identification of the discriminative capability of PCT and CRP to predict the development of a sepsis infection was done using ROC curves.
We compared the levels of PCT with CRP, which is an inflammatory marker known to increase in sepsis infections, using ROC curve to determine the performance of PCT in diagnosing sepsis. We identified that PCT has a better diagnostic ability with excellent AUC than CRP. In this study, the best cutoff point to identify bacterial infection was 0. 496 ng/ml for PCT with sensitivity of 96. 1% and specificity of 92. 9%. The AUC was 0. 968 (0. 928-1. 000) with a p value of 0. 000. The cutoff obtained for CRP in this study was 39. 281 mg/L and sensitivity of 56. 5% and specificity of 100%. The AUC for CRP was 0. 726 (0. 606-0. 847). Another study which aimed to use PCT to differentiate between bacterial infections and autoimmune disease recruited 397 patients and reported PCT to be a useful marker in identifying bacterial infections, recording PCT cutoff of 0. 512 ng/ml [20]. The high PPV of 98. 1% for PCT shows that PCT level of 0. 496 ng/ml predict 98. 1% chance bacterial infection and NPV of 100% shows that at a cutoff point of 0. 496 ng/ml, PCT concentration less than this predicts a 100% chance of no bacterial infection.
The AUC of PCT from this study, 0. 968. A study recorded AUC of 0. 911 and 0. 767 for PCT and CRP respectively in predicting septic shock which is in line with this research [21]. The results from this study (AUC of PCT and CRP recorded as 0. 968 and 0. 726 respectively) shows that PCT has a greater discriminative ability to identify the existence of bacterial infection than CRP though the AUC recorded for CRP is good, 0. 726 but it is lower as compared to that of PCT. Moreover, the cut-off point obtained for PCT in this study (0. 496 ng/ml) was similar to the clinical PCT reference value for diagnosing sepsis which is 0. 5 ng/ml.
Procalcitonin concentration was compared between patients with negative blood cultures and those positive blood cultures. There was a statistically significant difference (p=0. 001) between these groups, with the positive cultures group recording higher PCT levels showing that, procalcitonin increases in bacterial infections. This correlates with the clinical observations that, in bacterial infections which are confirmed with positive blood cultures, there is an increase in PCT [11].
Viral suppression was compared to PCT levels to identify any relationship between the two. There was a statistically significant difference between patients who had their HIV virus being suppressed and those who had viral suppression. But clinically, it has not being established that viral suppression affect PCT levels. There are no references to support this since this is the first study to establish a relation between viral suppression and PCT levels.
There was no significant difference between those who were on ART and those who were not on ART.
The findings of several studies have elaborated the association of PCT levels and patients’ survival. Septic patients with high PCT levels within few hours of hospital admission are known to be at a higher risk of dying as compared to those with low PCT levels.
In this study we recorded 89. 4% mortality rate representing 42 patients out of the 66 septic patients. These patients were immune compromised due to HIV/AIDS infection, coupled with co-infections present in some of the patients which could be attributable to some of the mortalities, sepsis is however accountable for most of the deaths. PCT levels were assessed in these patients and with a cut-off 0. 496 ng/ml predicting sepsis, a higher PCT level was determined among patients who died during in-hospital stay. There was a statistically significant difference (p=0. 000) in the PCT levels of patients who survived and those who died. In a research the AUC of PCT was 0. 72, they also reported a mortality rate of 55. 6% among septic patients with a statistically significant difference (p=0. 02) between survivors and non-survivors during in hospital stay with respect to PCT concentrations [5]. This supports our findings which shows that in patients with high levels of PCT, there is an increased of increased mortality.
Concentrations of PCT were high in the cases and very high in patients with positive blood cultures. PCT level was observed to significantly increase in patients who died during in-hospital stay. Concentrations of PCT corresponded with the severity of sepsis, with increasing PCT levels as sepsis became more severe. The sensitivity and specificity of PCT as a surrogate marker of sepsis in this study was 96. 1% and 92. 9% respectively. The AUC yielded for PCT at a cut-off of 0. 496 ng/ml was 0. 968, showing that PCT is a better marker of bacterial sepsis. This study showed that PCT is a better marker of sepsis which will help in diagnosis, prognosis and treatment monitoring.
Consent
As per the university standard, participants ’ oral or written consents where applicable were obtainedbefore enrollment into the study.
Ethical approval
Work received ethical approval from the Committee on Human Research and Ethics of College of Health Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
The authors are grateful to the study participants, the staff of Accident and Emergency Unit of Komfo Anokye Teaching Hospital, staff of the Molecular Medicine laboratory at Kwame Nkrumah University of Science and Technology, KNUST Research Fund and staff of Molecular Medicine Department, KNUST for their massive support in making this study a success.
Authors have declared that no competing interests exist.
Citation: Frimpong C, Agyemang-Yeboah F, Obirikorang C, Hardy Y, Nyame K, Acheampong I, et al. (2020) Procalcitonin as a Surrogate Marker of Sepsis in People Living with HIV/AIDS: A Case Study at Komfo Anokye Teaching Hospital, Ghana. J Clin Chem Lab Med. 3:143. DOI: 10.35248/clinical-chemistry-laboratory-medicine.20.3.143
Received: 11-Jun-2020 Accepted: 25-Jun-2020 Published: 28-Dec-2024 , DOI: 10.35248/2736-6588.24.7.297
Copyright: © 2020 Frimpong C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.