Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2017) Volume 5, Issue 1
L-Asparaginase appears to be highly effective especially in children with newly diagnosed Acute lymphoblastic leukemia. Prolonged L-Asparaginase intensification improved the outcome significantly. Nowadays L-Asparaginase is an essential drug that is used to treat children with Acute lymphoblastic leukemia all over the world. Its potential is now well established, as it has remarkably induced remission in most of the patients suffering with this disease. This therapy has brought a major breakthrough in modern oncology and with the development of its new functions, a great demand for L-Asparaginase is expected in the coming years. Keeping the potential of this particular enzyme in cancer treatment, the present work is planned with an intention of optimizing the production of L-Asparaginase both by conventional as well as by genetic engineering techniques. Pectobacterium carotovorum is selected for the present work based on its potentiality in cancer treatment and due to less glutaminase activity compared to the enzyme sourced from E. coli. Microbial culture was procured from MTCC, Chandigarh and screened for L-Asparaginase production in plate method and identified the activity based on the formation of pink color zone after overnight incubation.
Keywords: L-Asparaginase; Lymphoblastic leukemia; Pectobacterium carotovorum; Enzyme activity
Cancer is the most mystifying disease is defined as a disturbance of growth characterized primarily by excess proliferation of cells without apparent relation to the physiological demands of the organism involved. New technologies particularly high throughput screening, combinational chemotherapy, gene expression micro array and computer aided drug designing are increasing the speed and efficiency of drug development [1]. Improved systemic drug therapy is particularly important for the treatment of leukaemia, where surgery and radiation can no longer be curative. The development of microbial enzymes for cancer therapy, has added to the choice of anti-leukaemic drugs which promises prospects for better treatment. The use of microbial enzymes in leukemia therapy makes it important that the enzyme should be produced in large quantities and are usually required to be in their pure forms; therefore, their production costs are very high. If such a difficulty is circumvented, the potential use of enzymes in clinical medicine, in general and cancer chemotherapy in particular, would be extended.
The enzyme L-asparaginase has been a clinically acceptable antitumor agent for the effective treatment of acute lymphoblastic leukemia and lymphosarcoma. Acute leukemia is an uncommon malignant disorder resulting from the clonal proliferation of hematopoietic precursors of the myeloid or lymphoid lineages. Acute lymphoblastic leukemia is more common in children, while acute myelogenous leukemia predominates in adults. This is predominantly a disease of childhood and is the most common childhood cancer, accounting for 85% of childhood leukaemias [2]. The incidence of this disease is highest in the three to four year old age group, falling off by ten years. Generally the risk of any child developing acute leukaemia is about 1 in 2000. With modern chemotherapy 60%-70% of all children with acute lymphoblastic leukemia can be long-term survivors and are potentially cured. The chemotherapeutic potential of L-asparaginase in treating acute lymphoblastic leukemia and lymphosarcoma has been one of the most eminent discoveries of modern times. Children with, Acute lymphoblastic leukemia were found to be improved with more intensive combination chemotherapy from 4% in the early 1960s to more than 80% in the 1990s. The continuously improving treatment results through the years are an example of medical development and the handling of therapeutic schedules according to often international treatment protocols.
L-Asparaginase (l-asparagine amidohydrolase; EC 3.5.1.1) catalyzes the conversion of L-asparagine to L-aspartate and ammonia and to a lesser extent the formation of L-glutamate from L-glutamine. L-asparagine is a major requirement by the cells for the production of protein. It can be produced within the cell by an enzyme called asparagine synthetase or can be absorbed from the outside (consumed in the diet, absorbed into the body and made available to the body’s cells). Tumor cells, more specifically lymphatic tumor cells, require huge amounts of asparagine to keep up with their rapid malignant growth. Thus, the asparagines from the diet as well as what can be made by themselves (which is limited) is utilized by them to satisfy their large asparagine demand.
Microorganism and culture medium
The bacterial Strain Pectobacterium carotovorum (MTCC No. 1428) was used for the optimization work for the production of L-Asparaginase enzyme. The above mentioned bacterial cultures were procured from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India.
Nutrient medium
Nutrient broth and solid medium were used for fermentation as well as for propagation of Pectobacterium carotovorum strain. The medium was prepared with the following composition (gm/L) Beef extract 1.0, Yeast extract 2.0, Peptone 5.0, NaCl 5.0, Agar 15.0 and pH 7.0
Glucose Yeast Extract (GYE) medium
Glucose yeast extract medium (GYE) was used for the production of native L-Asparaginase, an antileukemic drug from wild strain of Pectobacterium. The medium was prepared as per the given composition (gm/L) K2HPO4-6, KH2PO4-3, NaCl-0.5, NH4Cl-1, Yeast Extract-5, Glucose-5, 1M MgSO4-2 ml, Trace elements-1 ml.
Composition of trace element
Trace elements were prepared as per the following composition (mg/L) Al2 (SO4) 3.7H2O-10, CuSO4.H2O-2, H3BO4-1, MnCl3.4H2O-20, NiCl2.6H2O-50, Na2MoO4.2H2O-50, ZnSO4.7H2O-50, FeSO4-50 and they were dissolved in double distilled water using HCL.
Determination of growth curve
For determination of four phases of growth curve, and to find the growth kinetics Pectobacterium carotovorum was grown in nutrient broth, nutrient broth with 2% Glucose, 5% Glucose, 2% Galactose, 2% Galactose with 0.1% Asparagine and as well as in Glucose yeast extract medium with 0.1% Asparagine and Galactose yeast extract medium with 0.1% Asparagine. The Experiment was carried out as per the given protocol.
a) Revived Pectobacterium bacterial culture is inoculated into 100 ml of nutrient broth medium and cultivated at 28 oC for overnight at 150 rpm, to prepare the seed culture.
b) 5% of the freshly gowrn culture was inoculated in to the above mentioned media and propagated the bacteria by keeping all the flasks at 28°C at 200 rpm.
c) The growth of the organism was determined at regular time intervals by measuring the absorbance at 600 nm and the medium without the culture was used as reference.
d) For determining the growth kinetics, it is required to have the dry cell weight of the biomass. For this, 1 ml of grown culture having 1 OD was transferred to a pre weighed 1.5 ml empty eppendorf tube and centrifuged at 7000 rpm for 15 min. Supernatant was discarded and dried the tube in incubator. The dried biomass was weighed and used to calculate the generation time and specific growth rate.
e) At the same regular time intervals the rate of the L-Asparaginase activity was also determined.
Screening of L-asparaginase producing organism
Pectobacterium carotovorum was screened for its ability of L-Asparaginase production as per the method described by Gulati. This is a novel and semi quantitative plate assay which measures the color changes of the medium around the bacterial colony producing L-Asparaginase in the presence of pH indicator phenol red [3]. The screening was carried out as per the following procedure.
a) Modified M9 medium was prepared as per the composition mentioned earlier.
b) The media was autoclaved and poured on to the sterilized petriplates.
c) The media was allowed to solidify and wells were made by making punches on the solidified medium.
d) 25 μl of grown culture of Pectobacterium carotovorum was added to the wells.
e) The plates were incubated for 16 hours at room temperature.
The production of L-Asparaginase, in general, results in increase of pH. Therefore the phenol red, which was usually yellow in color, turns to pink due to a shift from acidic to alkaline pH. This shift in pH, due to L- Asparaginase production was accompanied by the formation of a pink colored zone around the colonies.
Determination of L-asparaginase activity
The L-Asparaginase activity was determined by plotting a standard Ammonium sulphate curve by comparing the amount of ammonia liberated in the test samples.
Reagents
i) Tris buffer - 50 mM (pH 8.6 at 37° C)
ii) L-Asparagine - 189 mM
iii) Ammonium sulphate - 6 mM
iv) Trichloroacetic acid - 1.5 M
a) 0, 0.25, 0.5 and 1 ml volumes of 6 mM ammonium sulphate solution was taken in 2 ml micro centrifuge tubes and labeled as B, S1, S2, and S3 respectively.
b) 1 ml of 50 mM Tris buffer was added and the volume was made up to 1 ml by adding deionised water to each tube.
c) The tubes were incubated at 37°C for 30 minutes and were mixed by inversion at regular intervals of time.
d) 0.1 ml of 1.5 M trichloroacetic acid was added to all the tubes and vortexed immediately.
e) The tubes were centrifuged at 5000 rpm for 5 minutes.
f) 0.2 ml of supernatant was collected and was added to the test tubes containing 4.3 ml of deionised water.
g) To all the tubes 0.5 ml of Nesslers reagent was added and mixed thoroughly.
h) The absorbance was measured at 436 nm using the nonammonium sulphate solution (B) as reference.
Estimation of L-asparaginase activity
a) 1 ml of 50 mM Tris buffer and 0.1 ml of 189 mM L-Asparagine were taken in to 2 ml micro centrifuge tubes and marked as Test (T) and Blank (B).
b) The final volume in all the tubes was made up to 2 ml by adding deionised water.
c) The tubes were equilibrated to 37°C temperature.
d) 0.1 ml of cell suspension was added to the tubes marked as T.
e) The contents of the tube were mixed immediately by inversion and incubated at 37°C for 30 minutes.
f) 0.1 ml of 1.5 M trichloroacetic acid was added to all the tubes.
g) To the tubes marked as blank (B) 0.1 ml of cell suspension was added.
h) All tubes were centrifuged at 5000 rpm for 5 minutes to remove the protein precipitate.
i) 0.2 ml of supernatant was collected in the test tube containing 4.3 ml of deionised water.
j) 0.5 ml of Nesslers reagent was then added to the tubes and mixture was vortexed.
k) The absorbance was measured at 436 nm against a blank (B) solution as reference.
Standard curve:

A Standard curve was prepared by plotting the ?A436nm of the standard Vs Ammonia (NH3) concentration. And the standard graph denotes that 1 mole of ammonium sulphate corresponds to 2 moles of ammonia, therefore a 6 mM ammonium sulphate standard is equivalent to a 12 mM ammonium standard.
Sample determination:

The μ moles of ammonia (NH3) liberated was determined using the standard curve.
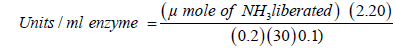
2.20=Total volume taken (ml); 0.2=Volume of the supernatant collected (ml); 30=Time of incubation (minutes); 0.1=Volume of cell extract (ml)
Unit definition: One International unit (IU) of L-Asparaginase is that amount of enzyme which liberates 1.0 μ mole of ammonium from L-asparagine per minute at pH 8.6 and 37°C temperature.
Factors effecting the enzyme activity: Factors like Substrate concentration, Time, Temperature, and pH were analyzed for their role in enzyme activity and to find optimal values for enzyme activity analysis.
Effect of substrate concentration
1. Eight clean and dry test tubes were taken and were divided into four sets viz., A, B, C, and D.
2. In each set, one test tube was marked as test (T) and the other as control (C). Different concentrations of substrate viz., 2.5, 5, 7.5, and 1 mg/ml of the standard substrate solution were added to four sets of tubes respectively. All other parameters like pH, time and temperature of incubation were kept constant.
3. Enzyme analysis was carried out as per the standard protocol descrined above, and readings were measured after addition of Nesslers reagent.
4. The difference in absorbance between the test and control gives the absorbance equivalent to asparaginase activity.
Effect of temperature
1. Eight clean and dry test tubes were taken and were divided into four sets viz., A, B, C, and D.
2. In each set, one test tube was marked as test (T) and the other as control (C). Parameters like pH, time and the optimal substrate concentration obtained in the earlier test were kept constant.
3. Enzyme analysis was carried out as per the standard protocol descrined above, by keeping the tubes at different temperatures for incubation viz., at 27, 32, 37, and 42°C.
4. Reaction was stopped by adding TCA and the tubes were centrifuged and further analysed for enzyme activity in all four sets of tubes to find the optimal temperature for maximal activity of the enzyme.
Effect of time on enzyme activity
1. Six clean and dry test tubes were taken and were divided into four sets viz., A, B, and C.
2. In each set, one test tube was marked as test (T) and the other as control (C). Parameters like pH, the optimal substrate concentration, and temperature obtained in the earlier tests were kept constant.
3. Enzyme analysis was carried out as per the standard protocol descrined above, by keeping the tubes for different intervals for incubation viz., for 15, 30 and 45 min.
4. Reaction was stopped by adding TCA and the tubes were centrifuged and further analysed for enzyme activity in all four sets of tubes to find the optimal temperature for maximal activity of the enzyme.
Effect of pH on enzyme activity
1. Eight clean and dry test tubes were taken and were divided into four sets viz., A, B, C and D.
2. In each set, one test tube was marked as test (T) and the other as control (C). Parameters like time, substrate concentration, and temperature were maintained at their optimal values obtained in the earlier tests.
3. Enzyme analysis was carried out as per the standard protocol descrined above, by adding the Tris buffer to maintain different pH range viz., 7.0, 7.5, 8.0, and 8.7 in all the four sets.
4. Reaction was stopped by adding TCA and the tubes were centrifuged and further analysed for enzyme activity in all four sets of tubes to find the optimal temperature for maximal activity of the enzyme.
Screening of L-asparaginase production
The chosen microorganism was screened for L-Asparaginase production by the method of Gulati. The appearance of pink colored zones on overnight incubation clearly says that the organism is having the ability of the required enzyme [4]. Results of the current study are partly in compliance with the earlier reports of Gulati.
Cornea has used same method of screening for L-Asparaginase while isolating and characterizing some L-Asparaginase producing recombinant Escherichia coli strains. Attempts were made to make a recombinant by the way of spheroplast fusion of preselected hyper producing local isolates of E. coli. Enzymatic activity 2-3 fold higher than the parental stains have been identified with this strategy.
Various organisms have been exploited so far for identification of potential source for large scale production of L-Asparaginase. In addition to the bacterial and fungal sources, few species of actinomycetes are also identified as potent producers of L-Asparaginase. As per the report of Sutthinan et al. a little modified method for screening of L-Asparaginase production from Actinomycetes. Actinomycetes were grown on yeast malt extract for 5 days and made 8 mm discs [5]. These were inoculated on Asparagine dextrose salts agar supplemented with phenol red and incubated at 28°C for 7 days. Plates were examined for pink zones and were considered as L-Asparaginase producing strains.
Determination of growth curve and growth kinetics
The revived bacterial culture was grown in various media to understand the growth curve, and growth kinetics viz., generation time and specific growth rate [6]. The media like Nutrient broth, and Nutrient broth with various combinations viz., 2% Glucose, 5% Glucose, 2% Galactose, 2% Galactose with 0.1% Asparagine, Glucose yeast extract medium with 0.1% Asparagine and Galactose yeast extract medium with 0.1% Asparagine.
The culture was grown up to OD is equal to 1, and the dry cell weight of the 1 OD culture determined as 8.8 mg/ml. This value was used to get the all dry biomass values and was considered for calculation of specific growth rate and generation time of the organism in various media combinations [7]. The specific growth rate and generation time of the organism in various media was tabulated in the Tables 1 and 2. From the results it is clearly understood that, the glucose is acting as catabolite repressor and inhibiting the growth as well as the enzyme production. Out of these 7 combinations, Galactose yeast extract medium with 0.1% asparagines was identified as the best one for attainting the maximal growth as well as the enzyme production. It is obvious that media sometimes can influence only the growth of the organism but not the product formation. In present work attempts have been made to design a medium composition having the role in growth as well as in production of L-Asparaginase. As per the reports of Sukumaran et al. the enzymatic activity was not observed in synthetic media compared to the semi defined medium for production of L-Asparaginase from mutant Serratia marcescens. It was identified that fructose, mannitol and sorbitol were the inhibitors for the enzyme production, maltose and Galactose were marginally favorable, and glucose and sucrose were the best carbon sources. These reports are not in compliance with the results obtained in the present study, where it was identified that the Galactose was the best carob source for growth as well as for the enzyme production.
| Substrate Conc. (mg/ml) | OD | mM | uM | U/ml |
|---|---|---|---|---|
| 25 | 0.065 | 1.477273 | 1477.273 | 5.416667 |
| 50 | 0.061 | 1.386364 | 1386.364 | 5.083333 |
| 75 | 0.043 | 0.977273 | 977.2727 | 3.583333 |
| 100 | 0.032 | 0.727273 | 727.2727 | 2.666667 |
Table 1: Effect of substrate concentration on Enzyme activity by P. carotovorum.
| Nutrient | Quantity g/l |
|---|---|
| K2HPO4 | 6 |
| KH2PO4 | 3 |
| NaCl | 0.5 |
| NH4Cl | 1 |
| Yeast Extract | 5 |
| Galactose | 20 |
| Aspargine | 1 |
| 1M MgSO4 | 2 ml |
| Trace elements | 1 ml |
Table 2: Composition of Galactose yeast extract medium used in Plackett-Burman design.
Determination of enzyme activity
The maximal activity phases were determined for the chosen organism by estimating the enzyme activity at the same regular intervals what were followed for growth kinetics. The growth pattern of the strain and enzyme activity relationships was determined and results had shown that there was a linear increase in the activity of L-Asparaginase along with the cell growth, and the results were represented in the Tables 3 and 4 (Figure 2). The maximal enzymatic activity phases were also found to be in coincidence with the maximum growth phases that is in the exponential phase of growth indicating the enzyme produced as a primary metabolite, but later decreased which might be due to exhaustion of nutrients, accumulation of by-products and feed-back inhibition of the enzyme. Recent studies with recombinant Escherichia coli and Erwinia carotovora have shown an enzymatic activity 2-3 fold higher than the parental strains, especially some of the recombinant Escherichia coli has shown a constant high level of L-Asparaginase biosynthesis [8-10]. In contrary our studies with the wild strains have shown comparatively low levels of enzymatic activity. This indicates that there is a need for rDNA technology to enhance the production levels of the enzyme.
| KH2PO4 | KH2PO4 | NaCl | NH4Cl | Yeast Extract | Galactose | Asparagine | 1M MgSO4 | Trace elements | |
|---|---|---|---|---|---|---|---|---|---|
| Run 1 | H | H | H | L | H | L | L | H | L |
| Run 2 | L | H | H | H | L | H | L | L | H |
| Run 3 | H | L | H | H | H | L | H | L | L |
| Run 4 | L | H | L | H | H | H | L | H | L |
| Run 5 | L | L | H | L | H | H | H | L | H |
| Run 6 | H | L | L | H | L | H | H | H | L |
| Run 7 | L | H | L | L | H | L | H | H | H |
| Run 8 | H | L | H | L | L | H | L | H | H |
| Run 9 | H | H | L | H | L | L | H | L | H |
| Run 10 | L | L | L | L | L | L | L | L | L |
Table 3: Plackett-Burman Media Design.
| Optimized parameter | Optimized condition |
|---|---|
| Temperature | 37°C |
| pH | 8.7 |
| Time | 30 min |
Table 4: Optimized conditions for L-Asparaginase activity by Pectobactrium carotovorum.
Factors effecting enzyme activity
For any enzyme analysis, the first one supposed to be optimized is the various factors like pH, Temperature, Time, and Substrate concentration.
Optimization of media by Plackett-Burman method
With the data available in the present work, it was understood that Galactose yeast extract medium with 0.1% Asparagine is the best medium composition for growth as well as for enzyme production. This was done as per the literature and random picking up the media and its constituents. But for constructive media optimization, the mathematical model derived by Plackett and Burman was chosen and carried out the experiment as per the described protocol in materials and methods [11]. Organism was grown in different sets of media which were designed as per the Placket Burman model. Various growth parameters like OD, Dry cell weight, and product formation in terms of activity were analyzed. Based on the results, it was identified as the flask no. 5 and 6 media composition was the optimal combination of nutrients suitable for the production of L-Asparaginase. This optimal media was chosen for further production studies.
There are many models available today for optimization of media components for obtaining the better yield of the product. In 2009, Kulanaisamy et al. have tried response surface methodology for production of L-Asparaginase by Serratia marcescens [12,13].
Experimental designs nowdays have been regarded as one of the most favorable techniques in covering a large area of practical statistics and obtain unambiguous results with the least expense. Response surface method designs help to quantify the relationships between one or more measured responses and the vital input factors. The most popular response surface methodologies are central composite, Box- Behnken designs. Box-Behnken design is an efficient and creative three level composite design for fitting second order response surfaces. It is an independent quadratic design [14]. The methodology is based on the construction of balance designs which are rotatable and enable each factor level to be tested several times. Each factor or independent variable can be placed at one of three equally spaced values (coded as -1, 0, and +1). In this design the treatment combinations are at the midpoints of edges of the cubical design region and at the center. Manikandan et al. have reported the usage of the experimental method of Box-Behnken designs for optimization of Asparaginase production by Pseudomonas aeruginosa and concluded that this is on the best models for optimization compared to Plackett Burman and central composite designs. The number of required experiments for this method is calculated according to N=k2+k+cp, where k is the factor number and cp is the replicate number of the central point. Latin square design was applied by Baskar et al. for evaluation and screening of nitrogen source for L-Asparaginse production by Aspergillus terreus.
Effect of substrate concentration
Four different substrate concentrations were taken and carried out the experiment as per earlier described protocol. Nesslerization was used to estimate the amount of ammonia liberated by the enzyme action and OD difference between the Test and Control was considered and a graph is drawn by taking the OD against to the substrate concentration. Based on the results it was clearly understood the maximum substrate concentration is required to obtain the maximal activity is 25 mg/ml (Table 1 and Figure 1).
Effect of temperature
Temperature is one of the most of important factor influences the rate of reaction of any catalyst. Out of these, biocatalysts are more sensitive for even to the minor changes in temperature. So, beyond the optimal temperature, there will no point of doing any enzyme analysis. Hence, to find the optimal temperature for the L-Asparaginase enzyme produced by Pectobacterium carotovorum, eight test tube set up was made and reaction was carried out as per the described procedure in the previous chapter. Results have shown that the temperature of 37°C is the most optimal one for analysis 37°C (Table 2 and Figure 3).
Effect of pH
L-Asparaginase enzyme produced by Pectobacterium carotovorum is also analyzed for its pH level, where it can show maximum activity [15]. Four pH ranges were maintained with the buffer as explained in the Materials and Methods. The pH 8.7 was identified as the optimal pH for the analysis of the enzyme activity (Table 3 and Figure 4).
Effect of time
Effect of time of incubation was also measured following the method described earlier. By that it is intended to keep the reaction till the last minute the existing enzyme in the reaction converts the best possible amount of substrate into product. From the data obtained in the present study, clearly says that the optimal time required for the reaction is 30 min (Table 4 and Figure 5).
The maximal enzyme activity of 20 U/ml was identified in Galactose yeast extract medium with 0.1% Asparagine and this medium is considered for further optimization. The optimized Galactose yeast extract medium with 0.1% Asparagine was taken for modeling by Plackett-Burman and as per the design 10 runs were carried out including dummy. Among all the 10 runs, the maximum enzyme activity of 26 U/ml was observed in run no. 5 and 6. By this, and also with the data from the random studies on media constituents, it was identified that Galactose and Asparagine were the key factors influencing the enzyme production.