Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2018) Volume 6, Issue 1
The NO oxidation process has been applied to improve a removal efficiency of NO included in exhaust gas. In this study, to produce a dry oxidant for the NO oxidation process, the catalytic H2O2 decomposition method was proposed. A variety of the heterogeneous solid-acidic Mn-based catalysts were prepared for the catalytic H2O2 decomposition and the effect of their physico-chemical properties on the catalytic H2O2 decomposition was investigated. The results of this study showed that the acidic sites of the Mn-based catalysts have an influence on the catalytic H2O2 decomposition. The Mn-based catalyst having the abundant acidic sites within the wide temperature range in NH3-TPD shows the best performance for the catalytic H2O2 decomposition. Therefore, the NO oxidation efficiency, using the dry oxidant produced by the H2O2 decomposition over the Mn-based catalyst having the abundant acidic properties under the wide temperature range, was higher than the others. As a remarkable result, the best performances in the catalytic H2O2 decomposition and NO oxidation were shown when the Mn-based Fe2O3 support catalyst containing K component was used for the catalytic H2O2 decomposition.
Keywords: Mn-based catalyst; Catalytic H2O2 decomposition; Dry oxidant; NO oxidation
NOx is a typical pollutant, which is produced through the combustion process of fossil fuels such as coal, petroleum, etc., and is discharged into the atmosphere [1]. Many researchers have conducted various studies to efficiently remove NOx, and a relatively widely applied technique is selective catalytic reduction (SCR) [2]. However, the selective catalytic reduction process requires a high temperature in order to increase the process efficiency while the catalyst is being applied. In the selective catalytic reduction process, NO is difficult to convert, which is a reason why a high temperature is required. Further, a process for oxidizing NO to NO2 or the like is required to relatively easily process the NO. In this study, the decomposition of H2O2 catalysts, which can produce dry oxidants for the oxidation process to convert NO to NO2, has been studied. Recently, a number of studies have been reported on various types of research results that can produce dry oxidants through H2O2 decomposition reaction on heterogeneous catalysts containing transition metals Fe and Zr as main components [3-5]. In addition, the H2O2 catalytic cracking reaction proceeds at a relatively low temperature to lower the operating temperature, and when the dry oxidant is applied to the oxidation process, the oxidation efficiency at a low temperature can be obtained [5]. It is believed that this simple facility can complement the disadvantages of the NO treatment process applied by the selective catalytic reduction method through the implementation of the process that can operate at low temperature with high efficiency [6-8]. In this study, H2O2 catalytic cracking was applied in order to convert the NO gas, which is difficult to process, into a dry oxidant that can be used simultaneously with the introduction of NO oxidation process. Therefore, the effect of the physico-chemical properties of the catalysts on the degradation of H2O2 catalysts was investigated by applying the catalysats having various physical properties in the H2O2 catalytic cracking process. The NO conversions characteristics were investigated. The reaction scheme for the production of dry oxidant by the decomposition of H2O2 using catalyst is shown in Equation (1), and the NO oxidation reaction formula is shown in (2) to (5) [9-11].
H2O2+M(II) →·OH+·OH (1)
NO+OH→HNO2 (2)
NO+OH→NO2+H (3)
NO+OH→HNO3 (4)
NO2+OH→NO3-+H+ (5)
Catalyst production
Figure 1 shows the process in which γ-Al2O3 is used as a carrier and the catalyst carrying Mn-based active material is prepared by the precipitation method. Mn(NO3)2 (reagent grade, Aldrich Co., 98% or more) was used as a precursor of the active material. A certain amount of Mn(NO3)2 was dissolved in distilled water in a beaker, and γ-Al2O3 as a carrier was mixed. Thereafter, precipitation process was carried out by dropping the NH4OH solution as a precipitant until the pH value of the slurry mixture prepared in the above was within the range of 9 to 10. The precipitated mixture in the slurry state was aged by stirring in a bath at about 80°C for about 4 h, then dried at 110°C for 24 h and fired at about 700°C for 4 h. The obtained Mn-based γ-Al2O3 catalyst, which is a solid product, was pulverized and the catalyst performance evaluation was carried out using a particle size ranging from about 100 to 150 μm through a sieving process. Figure 2 shows the preparation process of Mn-based catalyst with K component using Fe2O3 as a support. Fe2O3 (Powder, 4 (reagent grade, Aldrich Co., 98%) was used. First, the Fe2O3 support was mixed with distilled water through a mixing process to form a slurry state, and then mixed with a KMnO4 aqueous solution as a precursor of the active material. The mixture was then dried at 110 DEG C for about 12 h and then calcined at 700 DEG C for about 4 h to produce a Mn-based Fe2O3 support catalyst with a K component added thereto.
For the preparation of SO42-/ZrO2 catalyst, Zr (OC3H7)4 (reagent grade, Aldrich Co., 9 8% or more) was precipitated with ammonia water and dried at 110°C for 12 h. The dried precipitate was mixed with 1 N H2SO4 Aging for 12 h, drying at 120°C for 12 h, and calcining at 500°C.
Analysis of catalyst characteristics
The effect of various physical and chemical properties of various catalysts on H2O2 decomposition was investigated by various techniques as follows. XRD analysis was performed to investigate the crystal structure and properties of the prepared catalysts. The quantitative and qualitative analysis of the elements constituting the catalysts was performed using EDX (Energy Dispersive X-ray Spectrometer for FE-SEM, FISONS Co., LEO SUPRA 55, GENESIS 2000 (Carl Zeiss, EDAX), and resolution: 138 eV/5B-92U. X-ray photoelectron spectroscopy (X-ray photoelectron spectroscopy, K-Alpha model) was used to analyse the qualitative and quantitative analysis of the sample surface and the chemical bonding state of the constituent elements. The temperature programmed desorption (TPD) experiment using NH3 gas was conducted as follows to investigate the acidity characteristics of the heterogeneous catalysts prepared further. NH3 gas having a concentration of about 10 volume % is filled in the catalyst bed for 6 hours. After the adsorption process is proceeded, N2 gas is flowed and the temperature is increased from room temperature to 800°C at a rate of 5°C/min to be desorbed. The acid characterization was analysed by quantitative analysis of NH3 with a thermal conductivity detector (TCD). In order to investigate the decomposition characteristics of H2O2 catalysts, 55 g of H2O2 and 0.1 g of the prepared catalyst were reacted on the balance. The weight change of H2O2 over time was recorded in 2 s.
Decomposition of H2O2 and NO conversion experiment by type of catalyst
The experimental apparatus for evaluating the catalytic performance is composed of the H2O2 catalytic cracking process in which a dry oxidant is produced and the NO oxidizing process capable of oxidizing NO by injecting the dry oxidant prepared there from. The H2O2 catalytic cracking reaction, which can evaluate the catalytic performance, was carried out by passing H2O2 through an appropriate amount of flow rate together with the elevated temperature after a fixed amount of the catalyst was filled in the central portion of the tubular reactor of SUS material having an outer diameter of 1/2 inch. The reaction temperature was controlled through thermal transfer to the inside of the reactor using an automatic temperature controller after locating the heat conduction band (K-type) installed in the electric furnace at the same point as the catalyst filling layer. As the reactant, H2O2 (Duksan Co., 28-30%) to be decomposed was heated to be injected into the reactor in a vaporized state in the process of injecting the desired amount by using a metering pump. N2 was then transferred by using it as carrier gas and injected into the tubular catalytic reactor after mixing in a chambered mixer. The gas used in the experiment was adjusted to the experimental parameters using a calibrated MFC (mass flow controller, Linetech Co.). At the rear end of the reactor, a condenser and an adsorbent loading layer were used to remove moisture at the end of the heat line. The H2O2 decomposition efficiency (%) was calculated by substituting into equation 6. The system for the NO oxidation process, which is connected to the downstream of the H2O2 catalytic cracking process, was constructed so that the dry oxidant produced by the H2O2 decomposition and NO as the main reaction target can be simultaneously supplied at the desired concentration. In the NO oxidation process, a tubular reactor (material: SUS; outer diameter: 1/2 inch, length: 50 cm) filled with a fixed layer of bead type alumina having a diameter of 2.5 mm was used to increase the contact efficiency between the injected dry oxidant and NO gas ) Was used. Reactivity experiments were carried out by simultaneously injecting dry oxidant and NO gas produced by the decomposition of H2O2 catalyst into the tubular reactor. At this time, NO was used for the gas composition of 1,000 ppm and N2 was used as the dilution gas. The concentration values for the basic feed gas are shown in Table 1. The gas flow rate was controlled through a mass flow controller (Linetech Co.) with a calibrate d MFC. The reaction conditions are shown in Table 2. Reactants an d products were monitored by injecting every 5 min on-line to a gas chromatograph (DS science, Korea) equipped with a PDD (Pulsed Discharge Detector). In order to analyze NO and NO2 with G.C, a SUS separator filled with Hayesep Q was used. The detailed operating conditions for G.C are shown in Table 3. The NO conversion rate (% NO) to obtain the reactivity results was calculated by substituting the concentration of gases such as NO and NO2 before and after the reaction into the equation (7) as follows.
| Reactant gas | Composition |
|---|---|
| NO | 1,000 ppm |
| Balance gas: N2 | - |
Table 1: Basic composition in the gas bomb.
| Factors | Values |
|---|---|
| Temperature | 150°C |
| Space Velocity | 36,000 cm3/g-cat.∙h |
| Input NO concentration | 1,000 ppm |
Table 2: Basic reaction conditions for the catalytic H2O2 decomposition.
| Conditions | Values |
|---|---|
| Column material and length | Hysep Q (20 ft) |
| O.D. | 1/8 inch |
| Carrier gas, flow rate | He, 30 cm3/min |
| Column oven temperature | 40~110°C |
| Injector temperature | 110°C |
| Detector temperature | 110°C |
Table 3: Basic operation conditions of G.C.
 (6)
(6)
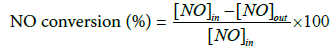 (7)
(7)
Characteristic analysis by catalyst
Table 4 shows the EDX analysis results for the constituents and their contents of K-Mn/Fe2O3, K-Mn/Al2O3, Mn/Al2O3 and ZrO2 catalysts prepared for H2O2 decomposition. The K-Mn/Fe2O3 catalysts with K and Mn contents of about 30 and 70 weight %. In support Fe2O3 had Mn contents of 5.3 and 10.7 at.%, Fe contents of 17.3 and 10.3 at.%, Respectively, K component is present at about 3.6 and 8.1 at.%. The Mn/ Al2O3 catalysts supported on Al2O3 support at 10, 30 and 70 weight %, Respectively, increased in the order of 3.5, 7.2 and 13.5% as the loading of Mn component increased. ZrO2 and SO42-/ZrO2 catalysts according to surface treatment by sulphuric acid show that the Zr component corresponding to about 25~26 at.% is formed irrespective of surface treatment and the S component was not detected because the content was very small even though it was surface-treated. The amount of acid sites corresponding to the total NH3 adsorption amount can be determined as an integrated value for a linear graph corresponding to the amount of desorbed NH3 that varies with temperature. The total amount of acid sites for the NH3 adsorption is Fe2O3
| Element | Content (at.%) | ||||||
|---|---|---|---|---|---|---|---|
| K-Mn/Fe2O3 | Mn/Al2O3 | ZrO2 | |||||
| 30wt.% | 70wt.% | 10wt.% | 30wt.% | 70wt.% | -- | sulfated | |
| Na | -- | -- | -- | -- | -- | -- | -- |
| Al | -- | -- | 16.5 | 13.2 | 10.1 | -- | -- |
| Mn | 5.3 | 10.7 | 3.5 | 7.2 | 13.5 | -- | -- |
| Fe | 17.3 | 10.3 | -- | -- | -- | -- | -- |
| K | 3.6 | 8.1 | -- | -- | -- | -- | -- |
| Zr | -- | -- | -- | -- | -- | 25.9 | 26.1 |
| O | 73.8 | 70 | 53 | 65.4 | 53 | 73.1 | 73.9 |
Table 4: EDX analysis of the various catalysts for the catalytic H2O2 decomposition.
| Catalysts | Peak No. | Max.Temp. (°C) | Quantity (mmol/g) | |
|---|---|---|---|---|
| K-Mn/Fe2O3 | 1 | 217.7 | 0.066 | |
| 2 | 297.3 | 0.372 | ||
| 3 | 355.5 | 0.212 | ||
| 4 | 399.3 | 0.062 | ||
| 5 | 452.7 | 0.225 | ||
| 6 | 689.9 | 0.109 | ||
| 7 | 762.9 | 1.337 | ||
| Mn/Al2O3 | 10 wt.% | 1 | 294 | 0.889 |
| 30 wt.% | 1 | 278.9 | 0.271 | |
| ZrO2 | 2 | 771.6 | 0.245 | |
| 1 | 293.5 | 0.138 | ||
Table 5: Acidic properties of the various catalysts by NH3-TPD.
Figure 8 shows the results of XPS analysis of the binding energy of elemental components to confirm the binding state of constituent elements of K-Mn/Fe2O3 catalyst supporting K and Mn in support Fe2O3. As shown in Figure 8a, the binding energy of the Mn component was obtained in various peaks, but at binding energies corresponding to about 642.2 and 654.1 eV. The type of compound containing Mn component corresponding to this binding energy position is KMnO4. Figure 8b also shows the binding energy of the K component, which is 292.6 eV, indicating the binding structure of K and KMnO4. The bond energy for the Fe component shown in Figure 8c was two peaks corresponding to about 710.05 and 723.5 eV, and this binding energy was found to be the binding energy of the Fe component contained in the Fe2O3 compound. Therefore, it is considered that the property of K-Mn/Fe2O3 catalyst is composed of Fe2O3 which is a support, mostly containing KMnO4 component. Figure 9 shows the results of XPS analysis of the binding energy of constituent elements for the 10 wt.% Mn/Al2O3 catalyst prepared by loading 10 wt.% Of Mn on the support γ-Al2O3. As shown in Figure 9a, the binding energy of the Mn component is about 641.8 and 653.8 eV, indicating that the main Mn compound with such binding energy is MnO2. The bond energy for the Al component contained in Al2O3, another component, was obtained at 73.9 eV as a single peak and is shown in Figure 9b. It can be seen that such a peak position is maintained as a support of the compound type introduced before production as the Al2O3 compound. Figure 10 shows the results of XPS analysis of the binding energy of the constituent elements for the 70 wt.% Mn/Al2O3 catalyst loaded with Mn on support Al2O3. Figure 10a shows a relatively large number of binding energy peaks as compared to the above-mentioned 10 wt% Mn-supported catalyst. As Mn content increases, various Mn compounds are formed in addition to MnO2 Able to know. However, the peaks corresponding to 641.7 and 652.8 eV were observed at the positions of the most important binding energies, respectively, and this binding energy tendency can be obtained as a result of the MnO2 compound. In Figure 10b, the binding energy position for the Al component is 73.6 eV, which means that it is present as a typical Al2O3 compound. Based on these results, it is considered that the Mn component is present in the MnO2 phase and the catalyst supported on Al2O3 is supported. As shown in Figure 11, the position of the peak corresponding to the bonding energy of the Zr component is 186.5 eV regardless of whether the surface treatment is performed or not. As can be seen in Figure 11, the peak position of the binding energy for the S component present in the SO42-/ZrO2 catalyst surface treated with sulfuric acid is about 168.8 eV. This means that SO42-functional groups are formed on the surface of ZrO2 due to surface treatment with sulfuric acid as binding energy corresponding to formation of SO42-functional group. These SO42-functionalized catalysts are known to enhance the catalytic effect in reactions involving related reaction mechanisms by further enhancing the strength and quantity of acid sites.
Decomposition of H2O2 and NO conversion according to the type of catalyst
Mn/Al2O3>10 wt.% Mn/Al2O3 catalyst was used as a catalyst. The results showed that the decomposition efficiency of H2O2 by the catalyst was K-Mn/Fe2O3 and Mn/Al2O3>Fe2O3>SO42-/ZrO2. The decomposition rate of H2O2 varies depending on the type of active material and the amount of dry oxidant generated at that time varies [13-15]. Figure 12 shows the conversion efficiency as H2O2 decomposition efficiency depending on the type of catalyst performed for the production of dry oxidant. These results are strongly influenced by the distribution of the acid sites of the catalysts, which are most related to the characteristics of the H2O2 decomposition reaction, among the physicochemical properties of the various catalysts produced. H2O2 decomposition reaction characteristics are dependent on the oxidation-reduction reaction due to electron mobility, and the reaction characteristic is related to the Lewis acid point of the solid catalyst. In addition, it is considered that the size of reactivity depends on the distribution and amount of weak acid sites in the characteristics of H2O2 as a reaction target in the acid sites. Figure 11 shows the amount of decomposition of H2O2 by decomposition of H2O2 catalyst by type of catalyst for the production of dry oxidant. The catalysts used were 10, 30, and 70 wt.% Mn/Al2O3 catalysts and SO42-/ZrO2 catalysts, which were different in content from K-Mn/Fe2O3 catalysts. As a result, it was found that the decomposition efficiency of H2O2 gradually increased with time regardless of the kind of catalyst, and it was found that the time required for stabilizing the decomposition efficiency of H2O2 catalyst, that is the order of magnitude of reactivity was as follows: K-Mn/Fe2O3>70 wt.% Mn/Al2O3>30 wt.% Mn/Al2O3>10 wt.% Mn/Al2O3>SO42-/ZrO2. The same tendency as the test result was obtained. To investigate the characteristics of the NO oxidation reaction proceeding in the downstream oxidation process by using the dry oxidant prepared according to the decomposition reaction characteristics of the performed H2O2 catalysts. Figure 12 shows the NO conversion efficiency (value in the graph, in%) by injecting the dry oxidant obtained after the H2O2 decomposition reaction carried out according to the above catalyst type into the oxidation process together with the exhaust gas. The reaction temperature was controlled at 150°C and the amount of H2O2 injected and the amount of catalyst loaded were 0.3 g/min and 0.5 g respectively. The oxidation process also controlled the reaction temperature to 150°C, and the flow rate of injected simulated flue gas and NO concentration were 300 ml/min and 1,000 ppm respectively. The conversion efficiency of NO in the oxidation process varied depending on the type of catalyst, and the NO conversion rate varied depending on the performance of the catalyst obtained in the H2O2 decomposition process. As a result, the NO conversion efficiency of the catalysts used in the H2O2 decomposition process was K-Mn/Fe2O3 and Mn/Al2O3 catalysts, and the conversion of NO obtained in the oxidation process reached about 100%. The NO conversion rates of the catalysts used in the H2O2 decomposition process were K-Mn/Fe2O3≈Mn/Al2O3>>30 wt.% Mn/Al2O3>10 wt.% Mn/Al2O3>Fe2O3>SO42-/ZrO2 And so on.
In this study, the conversion of dry oxidant obtained from H2O2 catalytic cracking was investigated by injecting the dry oxidant obtained from it into the subsequent NO oxidation process. First, various catalysts were prepared and applied to H2O2 decomposition reaction. As a result, it was found that the acid sites possessed by the catalyst had the greatest effect on the decomposition efficiency of H2O2, and the acid sites present on the catalyst surface were uniformly distributed from weak acid sites to strong acid sites. The H2O2 decomposition efficiency was higher. Also, when the dry oxidant obtained from this process was injected into the NO oxidation process, the NO conversion efficiency was increased as the decomposition efficiency of H2O2 was increased. Therefore, Mn-based Fe2O3 catalyst with K component as the catalyst having a uniform and rich acid point ranging from the weakest point to the strongest point among the various catalysts produced showed the highest decomposition efficiency of H2O2, The NO conversion rate obtained under optimized operating conditions on the NO oxidation process using dry oxidant reached about 100%.
This study was supported by the Ministry of Environment’s environmental industry advancement technology development project.