Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Review Article - (2022)Volume 12, Issue 5
Crystalline non-covalent Inclusion Compounds (ICs) have been formed between many guest polymers and several small host molecules, including among them Cyclo-Dextrins (CDs) and Urea (U) hosts. In these ICs, the guest polymer chains are included in the narrow host crystalline lattice channels (0.5-1.0 nm) and are separated and highly extended. Upon the careful removal of the host lattice the guest polymers are coalesced into bulk samples that are organized differently from bulk samples obtained from their solutions and melts. Likely, because such coalesced polymers are more extended and less entangled, they evidence distinct behaviour and properties, which surprisingly are highly resistant to annealing above their Tgs and Tms. Coalesced polymers allow the production of materials with unique behaviours, two types of which are the subject of this review chapter: 1. Single polymer composites formed from as-received and coalesced samples of the same polymer and 2. Intimately mixed blends formed by the coalescence of two or more different polymers from their common ICs. In addition to small molecule additive ICs, both of these coalesced polymer materials can be potentially utilized in a variety of medical applications, some of which are described here.
Coalesced polymers; Cyclodextrin hosts; Single polymer composites; Compatible polymer blends
Over 30 and 50 years ago, respectively, the formation of noncovalently bonded inclusion compounds formed between Cyclo- Dextrins (CDs) and Urea (U) hosts and guest polymers were reported [1-3]. Additional hosts that form ICs with polymer guests are Thio-Urea (TU) [1,2] Perhydotriphenylene (PHTP) [4-6], and Cyclo-Triphosphazines (TPP) [7]. Figures 1-3 illustrate the channels found in the host U, TPP, and CD-IC clathrates [8,9], where the extended and isolated guest polymer chains are included.
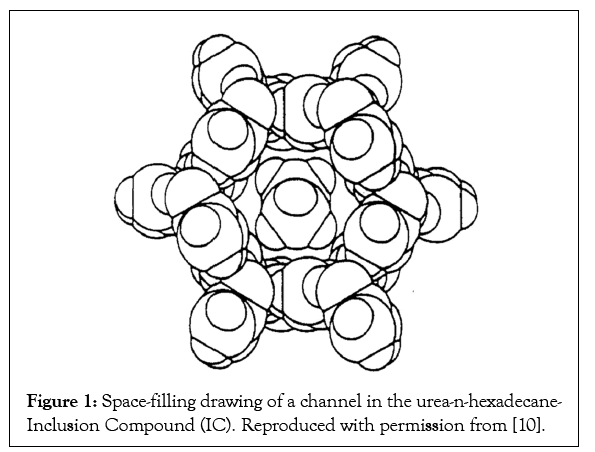
Figure 1: Space-filling drawing of a channel in the urea-n-hexadecane- Inclusion Compound (IC). Reproduced with permission from [10].
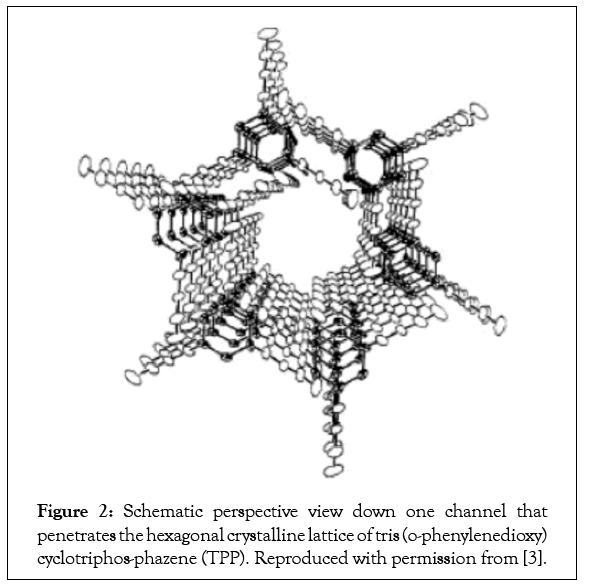
Figure 2: Schematic perspective view down one channel that penetrates the hexagonal crystalline lattice of tris (o-phenylenedioxy) cyclotriphos-phazene (TPP). Reproduced with permission from [3].
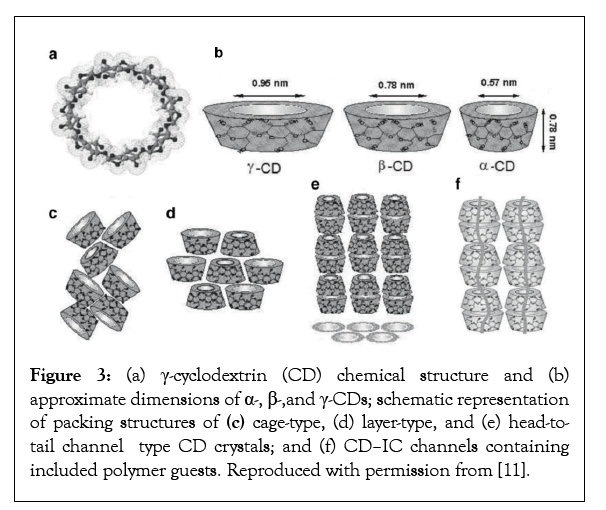
Figure 3: (a) γ-cyclodextrin (CD) chemical structure and (b) approximate dimensions of α-, β-,and γ-CDs; schematic representation of packing structures of (c) cage-type, (d) layer-type, and (e) head-totail channel type CD crystals; and (f) CD–IC channels containing included polymer guests. Reproduced with permission from [11].
In the case of polymer-CD-ICs, as the host cyclic sugars thread over the guest polymer chains the small numbers of included water molecules residing there are replaced by the included polymer [8,9]. Because the interiors of CDs are largely hydrophobic, replacement of hydrophilic water by more hydrophobic guest polymers is thought to favor IC formation [8,9], along with hydrogen bonding and van der Waals interactions [10]. The latter are short-range interactions favored by close proximity of host and guest molecules and suggest that a tight fit of the included guest polymer with the walls of the host channel are important to the stability of polymer-ICs made with all host clathrates.
Clearly guest polymers included in the narrow channels of their ICs are highly extended and separated from neighbouring chains by the host crystalline lattice (Figures 1-3). Such included polymers can have conformations and mobilities quite distinct from their bulk samples [11,12]. Also when the crystalline lattices are carefully removed, the resulting coalesced guest polymers are apparently reorganized and evidence behaviors and properties distinct from their solution and melt processed bulk samples [11,13-20]. For example, Figures 4 and 5 illustrate, respectively, that polymer samples coalesced from their ICs have higher glass transition temperatures (Tgs) and enhanced crystallizabilities [11,13-16].
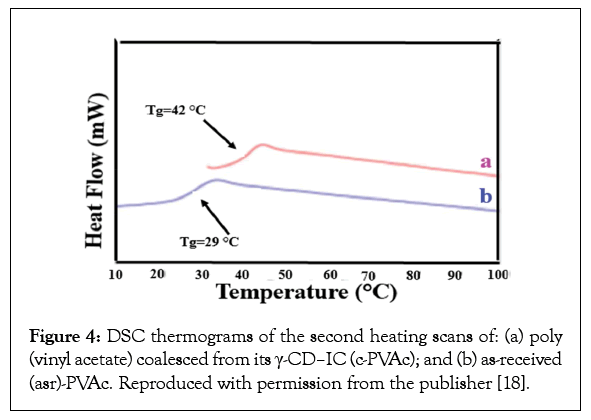
Figure 4: DSC thermograms of the second heating scans of: (a) poly (vinyl acetate) coalesced from its γ-CD–IC (c-PVAc); and (b) as-received (asr)-PVAc. Reproduced with permission from the publisher [18].
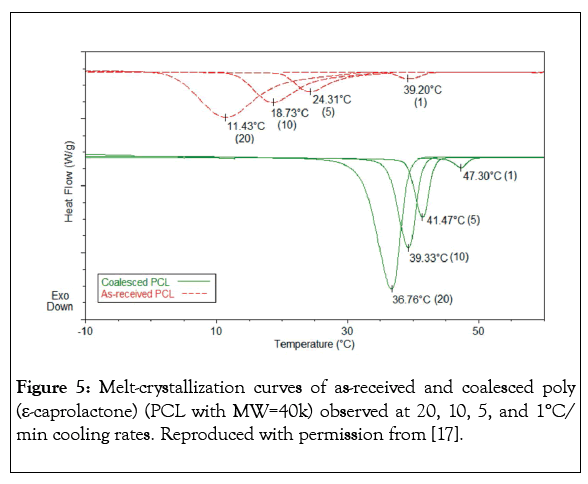
Figure 5: Melt-crystallization curves of as-received and coalesced poly (ε-caprolactone) (PCL with MW=40k) observed at 20, 10, 5, and 1°C/ min cooling rates. Reproduced with permission from [17].
Surprising and of possible commercial importance was the discovery that the unique behaviours’ and properties of polymer samples coalesced from their ICs are resistant to high temperature annealing well above their Tgs and melting temperatures (Tms). Such behaviour is illustrated for c-PVAc in Table 1 and Figure 6 and for c-PCL in Table 2.
| Annealing time (days) | Tg (°C) |
|---|---|
| 0 | 41 |
| 2 | 41.7 |
| 8 | 41.5 |
| 14 | 41.2 |
Table 1: Tgs of c-PVAc obtained from its γ-CD-IC annealed above Tg at 70°C for different times. Adapted with permission from [18]. Copyright Gurarslan, et al. North Carolina State University 2017.
| Melt crystallization temperatures | |||
|---|---|---|---|
| A. | Tc (°C) | Tc increments (°C) | |
| Sample | asr-PCL | c-PCL | Tmc (c-PCL)-Tc (asr-PCL) |
| PCL-2 | 17 | 26.5 | 9.5 |
| PCL-10 | 16.8 | 32.5 | 15.7 |
| PCL-40 | 18.8 | 35 | 16.2 |
| PCL-80 | 11.7 | 33.3 | 21.6 |
| After Annealing c-PCL for 2 and 4 weeks at 80 °C | |||
| B. | Tc (°C) | ||
| Sample | Initial | annealed for 2 weeks | 4 weeks at 80°C |
| c-PCL-2 | 26.5 | 26 | 6.4 |
| c-PCL-10 | 32.5 | 30.9 | 31.7 |
| c-PCL-40 | 35 | 34.1 | 33.8 |
| c-PCL-80 | 33.3 | 30.1 | 31.1 |
Note: *PCLs with molecular weights of 2, 10, 40, and 80 kg/mole
Table 2: Melt crystallization temperatures of asr- and c-PCLs (from their U-ICs) with different molecular weights*-A. asr- vs. c-PCLs and B. c-PCLs Annealed in their melts [19].
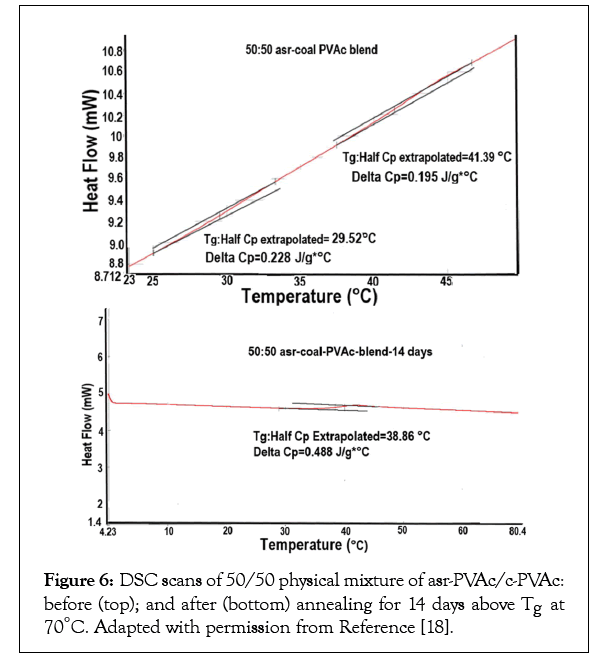
Figure 6: DSC scans of 50/50 physical mixture of asr-PVAc/c-PVAc: before (top); and after (bottom) annealing for 14 days above Tg at 70°C. Adapted with permission from Reference [18].
Single polymer composites
We can take advantage of the more crystallizable c-polymers by using them as self-nucleants to increase the melt crystallizability of the same asr-polymers. Table 3 presents the crystallization temperatures of asr, c, self-nucleated and nucleated (with PCL-10) PCL samples with different molecular weights. The Tcs of the c-and nucleated-PCLs are significantly higher than the asr-PCL samples.
| Tc(°C)s | ||||
|---|---|---|---|---|
| Asr | C | Self Nuc-PCL | Nucleated with Nuc-PCL-10 | |
| Sample | ||||
| PCL-2 | 17 | 26.5 | 18.8* | 21.3* |
| PCL-10 | 16.8 | 32.5 | 27.9 | 27.9 |
| PCL-40 | 18.8 | 35 | 32.3 | 29.5 |
| PCL-80 | 11.7 | 33.3 | 29.6 | 29.3 |
Note: *Nucleated PCL samples contain 2.5 wt% of the PCL nucleant and 97.5 wt% of asr-PCLs
Table 3: Crystallization temperatures of different molecular weight asr-PCLc, c-PCLc, self-nuc-PCLs, and PCLs nucleated with c-PCL-10 [19].
Figure 7 makes plain that self-nucleation of the melt crystallization of polymers with small amounts of the same c-polymer is quite practical [20]. The reason for this is due to the fact that after the first nucleation using c-polymers the resultant nuc-polymer is also rapidly crystallizable and may be used to nucleate additional asrpolymer samples. Such self-nucleated polymer materials have the advantage that they contain nothing but the same polymers as the original asr-sample. As a consequence, they are more easily recycled and contain no additives that would preclude their implantable medical uses. Much more polymer chains are included in U-ICs than are contained in their CD-ICs. As a result, multiple grams of c-PCL were easily obtained from their U-ICs, (See Figure 8) and were melt-spun into monofilament fibers [21]. The mechanical performances of the as-spun and drawn fibers are summarized in Table 4. There it is apparent that the as-spun and drawn c-PCL monofilaments are significantly stronger and less deformable than their asr-PCL fiber counterparts.
| Fibers | asr-PC | c-PCL | asr PCL | Drawn c-PCL |
|---|---|---|---|---|
| Modulus (MPa) | 41 ± 6 | 271 ± 20 | 465 ± 12 | 770 ± 32 |
| Elongation at break (mm) | 197 ± 19 | 110 ± 8 | 32 ± 2 | 14 ± 2 |
| % crystallinity | 40.6 | 50.1 | 50.8 | 53.3 |
Table 4: Crystallinity and Mechanical Properties (Mean ± Standard Error) of Asr- and c-PCL melt-spun monofilament fibers [21].
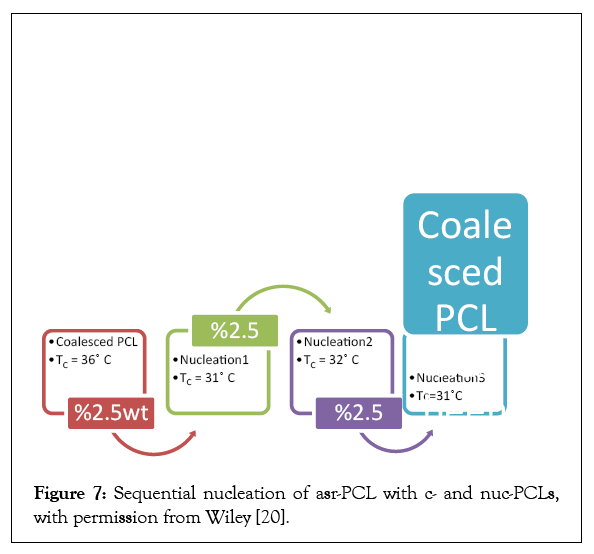
Figure 7:Sequential nucleation of asr-PCL with c- and nuc-PCLs, with permission from Wiley [20].
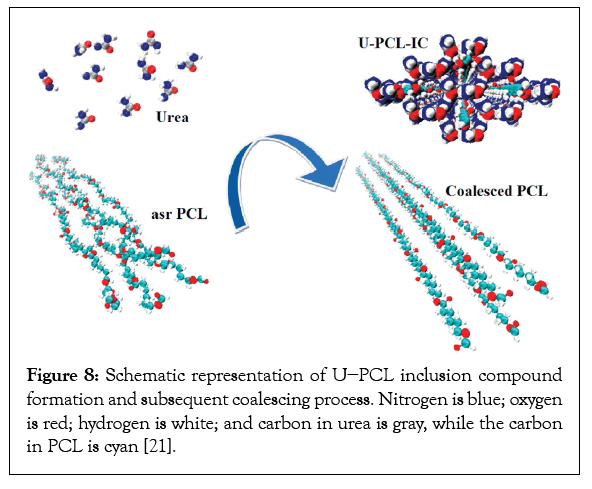
Figure 8: Schematic representation of U−PCL inclusion compound formation and subsequent coalescing process. Nitrogen is blue; oxygen is red; hydrogen is white; and carbon in urea is gray, while the carbon in PCL is cyan [21].
Melt-spinning c-polymers is not practical because the yield of polymers coalesced from their ICs is relatively small. On the other hand, it will soon be demonstrated that melt spinning of fibers made from asr-polymers initially self-nucleated with the same c-polymer are mechanically superior to their melt-spun unnucleated asr-polymer fibers. Such superior self-nucleated fibers may be easily and practically manufactured. In Table 5 we compare the densities and permeabilities of asr-Poly Ethylene Terephthalate (PET) and self-nuc-PET films. As we have just described above and demonstrated (Tables 2 and 3), (Figure 5) polymers coalesced from their CD-and U-ICs show increased abilities for crystallization and may self-nucleate the crystallization of the same asr-polymers when added in small amounts. Such coalesced polymers also exhibit improved mechanical properties, as evidenced above in Table 4 and below in Table 6 and Figure 9a. Their self-nucleated films also exhibit improved mechanical properties as illustrated in Figure 9a.
| PET samples | asr-PET | nuc-PET |
|---|---|---|
| Sample density at 25°C (g/cm-3) | 1.368 | 1.386 |
| Permeability (P × 1014 ) (cm3 s-1Pa-1) | 1.64 | 0.57 |
Table 5: Densities and CO2 (0.2 MPa) permeabilities in PET films [22].
| Sample | Modulus (MPa) | Elongation at Break (mm) |
|---|---|---|
| asr/asr-PCL 80-film | 317.5 ± 24 | 270.3 ± 30 (540%) |
| asr/nuc-PCL 80-film | 340.7 ± 28 | 253.6 ± 15 (507%) |
| asr/asr-PCL 70–90 | 217 ± 24 | 415 ± 52 (830%) |
Table 6: Tensile test results for bilayer PCL films obtained with permission from [23], copyright 2015 American chemical society.
| Sample | Average thickness (mm) | Water vapour permeability (g/m2/24 h) |
|---|---|---|
| PCL film | 0.022 | 375 |
| dipped PCL film | 0.01 | 440 |
| PC-Urea film | 0.139 | 413 |
| Dipped PCL-urea film | 0.313 | 747 |
| PCL-IC film | 0.054 | 418 |
| Dipped PCL-IC film | 0.076 | 583 |
| PLLA film | 0.024 | 173 |
| Dipped PLLA film | 0.041 | 187 |
| PLLA-urea film | 0.18 | 207 |
| Dipped PLLA-urea film | 0.155 | 540 |
| PLLA-IC film | 0.045 | 183 |
| Dipped PLLA-IC film | 0.052 | 236 |
Table 7: Thicknesses and moisture vapor permeabilities of neat and U- or PCL-U- IC embedded PCL and PLLA films before and after dipping in Methanol. Obtained with permission from [26]. Copyright 2001 WILEY.
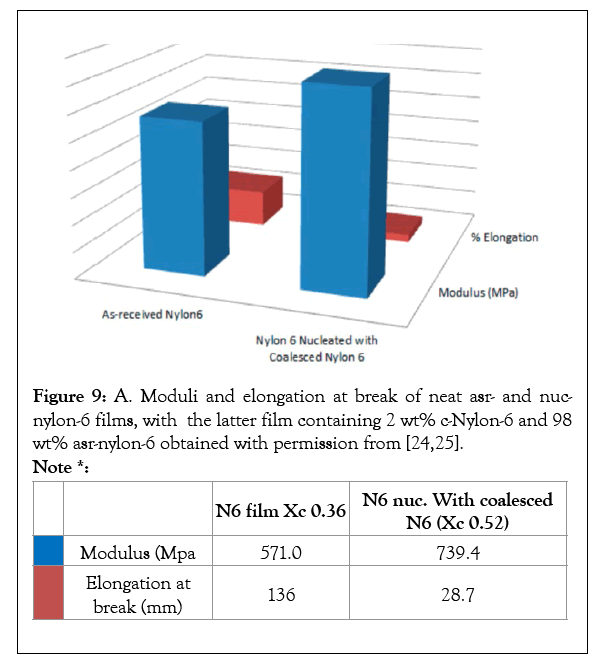
Figure 9a: A. Moduli and elongation at break of neat asr- and nucnylon- 6 films, with the latter film containing 2 wt% c-Nylon-6 and 98 wt% asr-nylon-6 obtained with permission from [24,25].
Note *:
| N6 film Xc 0.36 | N6 nuc. With coalesced N6 (Xc 0.52) | ||
|---|---|---|---|
 |
modulus (Mpa | 571.0 | 739.4 |
 |
elongation at break (mm) | 136 | 28.7 |
Single polymer composites may be fabricated with a combination of As-Received (asr) and Self-Nucleated (nuc) samples of the same polymer [22-25]. The asr-and nuc-films were melt pressed into a sandwich and held together for 10 min above their Tms. The mechanical properties of two-layer sandwich films of Asr-/asrand asr-/nuc-nylon-6 are presented in Figures 9b and 10, where it is observed that the asr-/nuc-PCL and nylon-6 sandwiches are stronger and less easily plastically deformed. In addition, T-Peel tests were performed on the asr-/nuc-PCL film sandwiches with no observed delamination [23]. We have also made polymer A-polymer B composites via embedding a polymer A-U-or -CD-IC into polymer B fibers or films, which was followed by washing with a solvent for U or CD to remove the embedded host and release and coalesce the included polymer A [26].
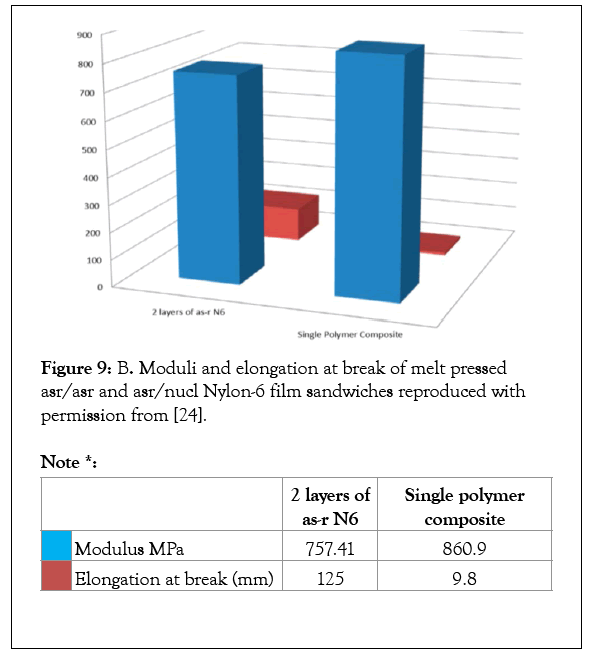
Figure 9b :B. Moduli and elongation at break of melt pressed asr/asr and asr/nucl Nylon-6 film sandwiches reproduced with permission from [24].
Note *:
| 2 layers of as-r N6 | Single polymer composite | ||
|---|---|---|---|
 |
modulus MPa | 757.41 | 860.9 |
 |
Elongation at break (mm) | 125 | 9.8 |
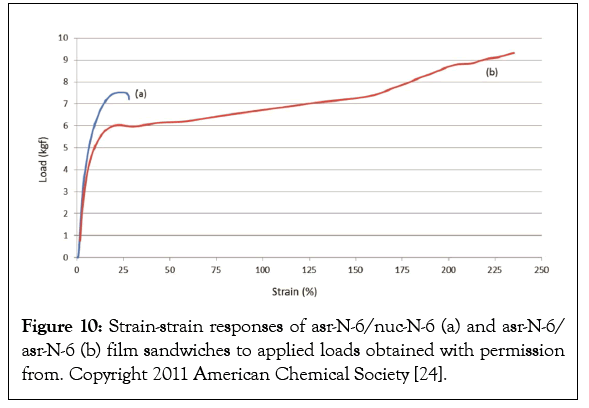
Figure 10: Strain-strain responses of asr-N-6/nuc-N-6 (a) and asr-N-6/ asr-N-6 (b) film sandwiches to applied loads obtained with permission from. Copyright 2011 American Chemical Society [24].
DSC observations of such polymer A (PCL)-polymer-B (PLLA) composites are presented in Figure 11 [26]. In (a) and (c) we observe the melting of the PCL-U-IC at higher and lower temperatures, and the melting of PCL and PLLA at the lower temperatures. There are no DSC endotherms for PCl-U-IC or free U in DSC scans (b) and (d). Rather only melting of neat PCl or neat PLLA are observed. While the melting of coalesced PCL is is not observed in DSC scan (d), the presence of a small amount of c-PCl was observed, though not presented here, by solid-state 13C-NMR [26]. In Table 7 are presented water vapour permeability’s of pure U and PCL-U-IC and the PCL-U-IC embedded PCl and PLLA films before and after washing with methanol. Presumably removing the U crystals of the U-embedded PCL ad PLLA films produces holes, leading to large observed increases in their water vapour permeabilities.
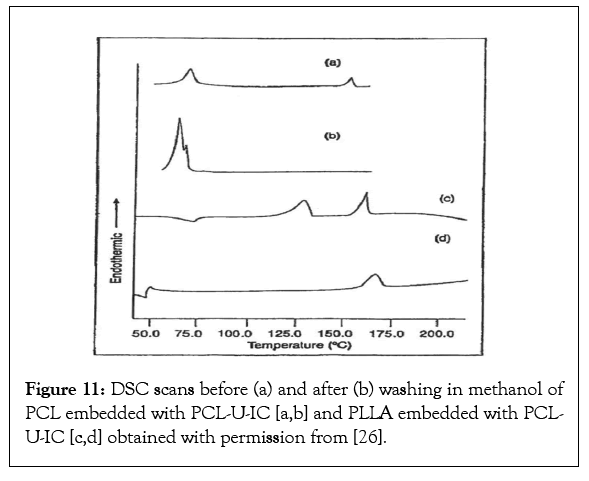
Figure 11: DSC scans before (a) and after (b) washing in methanol of PCL embedded with PCL-U-IC [a,b] and PLLA embedded with PCLU- IC [c,d] obtained with permission from [26].
After soaking in methanol, the films embedded with PCL-U-IC do not evidence significant increases in permeabilities, suggesting that any holes created by the removal of the host U from its PC-IC crystals upon soaking in methanol are filled in by the coalesced PCL. For PLLA and nylon-6 films embedded with the α-CD–IC of poly (ethylene oxide), similar results were observed before and after washing in hot water [26]. Possible reasons for the failure of reorganized polymer samples coalesced from their crystalline ICs to return to their randomly-coiling and entangled organization when annealed at temperatures well above their Tgs and Tms have been offered [15,27,28]. Though the largely extended, separated, and un-entangled chains coalesced from a single crystal in the polymer- IC crystalline powder (Figure 12) likely random-coil relatively rapidly, the center-of-mass diffusion that must accompany full entanglement of all the chains in the sample is extremely sluggish.
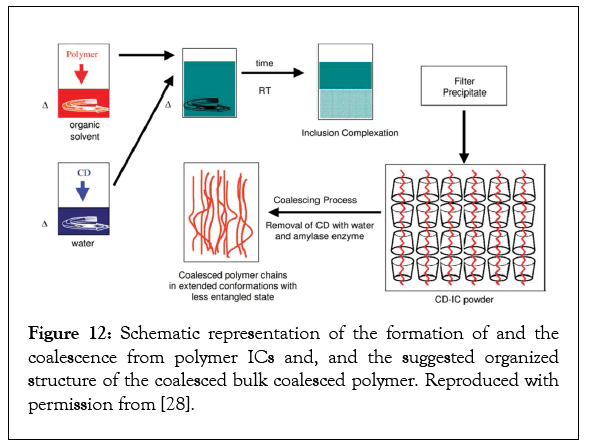
Figure 12: Schematic representation of the formation of and the coalescence from polymer ICs and, and the suggested organized structure of the coalesced bulk coalesced polymer. Reproduced with permission from [28].
The process of entangling the largely separated and not fully interpenetrating randomly coiled chains coalesced from their CD–IC crystalline powders is apparently particularly slow. In fact much slower than the center-of mass diffusion of polymer chains in their fully entangled melts for an apparently analogous slow restructuring observed in the melting of single crystals of Ultra- High Molecular Weight Polyethylene (UHMWPE) [29] (Figure 13).
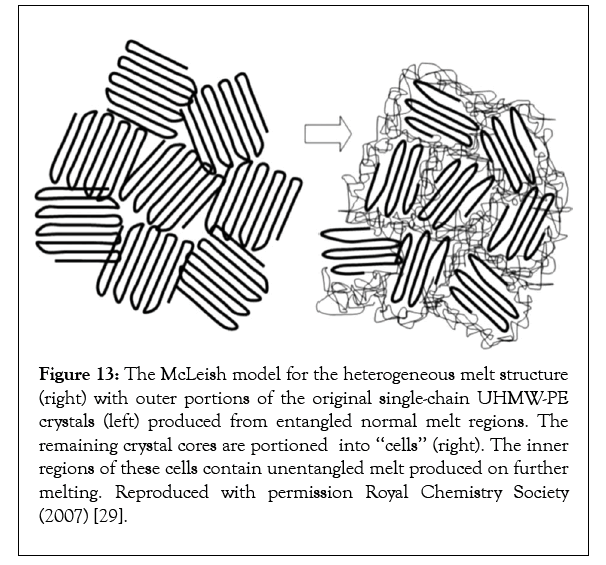
Figure 13: The McLeish model for the heterogeneous melt structure (right) with outer portions of the original single-chain UHMW-PE crystals (left) produced from entangled normal melt regions. The remaining crystal cores are portioned into ‘‘cells’’ (right). The inner regions of these cells contain unentangled melt produced on further melting. Reproduced with permission Royal Chemistry Society (2007) [29].
Miscible blends from common polymer ICs
We have also made a large number of miscible homopolymer blends using the formation of and coalescence from CD-ICs containing two or more ordinarily immiscible guest polymers [30]. This is illustrated in Figure 14 by removal in this case of the host CD crystalline lattice from the common ICs containing two or more included guest polymers. Well-mixed polymer blends, including Poly(L-Lactic Acid) (PLLA)/PCL [31,32], Polycarbonate (PC)/Polystyrene (PS) or or Poly (methyl methacrylate) (PMMA) [33], Atactic-Poly(Β-Hydroxy Butyrate) (PHB)/PCL [34], PET/ Poly(Ethylene 2,6-Naphthalate) (PEN) [35], PC/PMMA [36], PVAc/ PMMA/PC [37], PVAc/PMMA [33,36] or PC [36], and nylon-6/ nylon-6,6 [38], were made for these inherently immiscible polymer blends upon coalescence from their common CD-ICs. Discussion of well-mixed blends obtained by coalescence from their common CD–ICs will be restricted to the PLLA/PCL [30,31] and Isotactic Polypropylene (i-PP)/ Isotactic Poly(1-butene) (i-PB) Binary Blends, whose behaviours’ are representative of all those listed above.
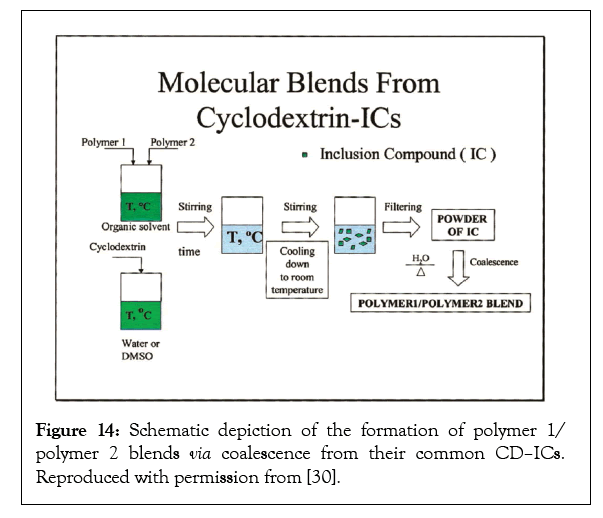
Figure 14: Schematic depiction of the formation of polymer 1/ polymer 2 blends via coalescence from their common CD–ICs. Reproduced with permission from [30].
Figures 15a-15d presents polarized micrographs of melt pressed films of PLLA (a) and PCL (b), a solution cast film (c), and a meltpressed film of PLLA/PCL coalesced from its common α-CD-IC (d). A lack of and substantial mixing of PLLA and PCL, respectively, is apparent by the comparison of the solution-cast and melt-pressed coalesced PLLA/PCL films.
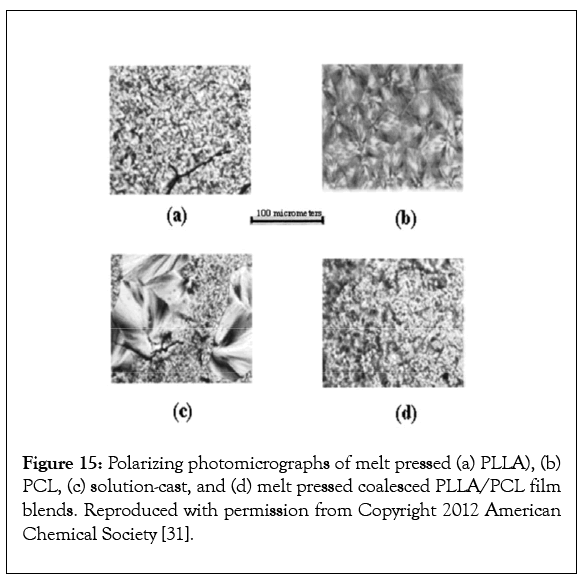
Figure 15: Polarizing photomicrographs of melt pressed (a) PLLA), (b) PCL, (c) solution-cast, and (d) melt pressed coalesced PLLA/PCL film blends. Reproduced with permission from Copyright 2012 American Chemical Society [31].
The 2D-1H-13C Heteronuclear Correlation (HETCOR) NMR spectra of the solution-cast and coalesced PCL/PLLA blends observed with short (50 μs) and long (1.0 ms) mixing times [39-41] are shown in Figure 16. With the shorter mixing time a normal 1H–13C correlation spectrum is observed for both coalesced and solution-cast blends, where the PLLA methyl and PCL methylene proton chemical shifts are clearly distinguished. In the HETCOR spectrum observed with the longer mixing time for the coalesced PCL/PLLA blend, these two proton chemical shifts approach each other. This observed effective spin-diffusion requires spatial proximity between the PLLA and PCL protons, strongly suggesting intimate mixing in the coalesced PCL/PLLA blend [36,39].
The HETCOR spectrum of the solution-cast blend does not show significant proton spin-diffusion between PCL and PLLA chains even when a long mixing time is employed. Clearly then intimate mixing and phase separation, respectively, in the coalesced and solution cast PCL/PLLA blends are revealed by efficient proton spin-dffusion in the former blend and the absence of spindffusion in the latter blend. The intimacy of mixing in the largely amorphous PCL/PLLA blends produced by coalescence from their α-CD–IC crystals and those cast from their common solution were estimated [39]. This was achieved through use of a two-dimensional HETCOR spin-diffusion technique [39-43], which enabled spin-diffusion coefficients and the length scales of miscibility to be determined by direct measurement. Length scales of mixing in the coalesced and solution cast PCL/PLLA blends were found to be 4.9 and 7.4 nm, respectively. The radii of gyration for the PCL and PLLA chains investigated were expected to be 3.5–5.0 nm, which are consistent with the length scale of mixing in the coalesced blend.
The length scale of mixing in the solution cast PCL/PllA blend (7.5 nm) exceeds the radii of gyration of both polymers. Only the PCL and PLLA chains in the coalesced blend are molecularly mixed.
Though PCL and PLLA are both biocompatible and biodegradable neither possesses favourable mechanical performance. PCL has low Tm and tensile strength. PLLA’s Tm is much higher, but it has low ductility and is brittle. They are inherently incompatible with each other, as we have seen in their solution cast blend (Figures 15a-15d). Even after heating well above their TmS at 200°C for 12 h, in the blend coalesced from its common α-CD–IC, the PCL and PLLA chains remain intimately mixed [31].
A 1.6:1.0:1.4 molar PC/PMMA/PVAc ternary polymer blend obtained by coalescence from their common α-CD-IC appeared to be well-mixed [37]. The first and second heat DSC scans presented in Figure 17 evidence a single Tg at 57°C, consistent with the formation of an intimate PC/PMMA/PVAc ternary blend. It should be mentioned that plotting the reversing heat flow versus temperature removed the effect of trace solvent release, as well as the enthalpic recovery of an endothermic peak that occurred just above the Tg. Co-precipitation from a common tetrahydrofuran solution showed four distinct Tgs at 38, 56, 82 and 133°C. These correspond to separate PVAc, PMMA/PVAc, PMMA, and PC phases. Also note the appearance of only one glass transition in the second heating of the coalesced ternary blend that confirms the stability of the intimate molecular mixing of PC, PMMA, and PVAc chains in their coalesced ternary blend.
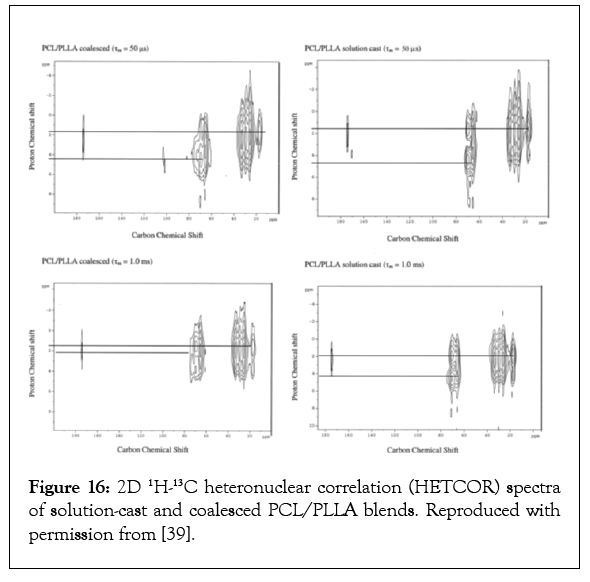
Figure 16: 2D 1H-13C heteronuclear correlation (HETCOR) spectra of solution-cast and coalesced PCL/PLLA blends. Reproduced with permission from [39].
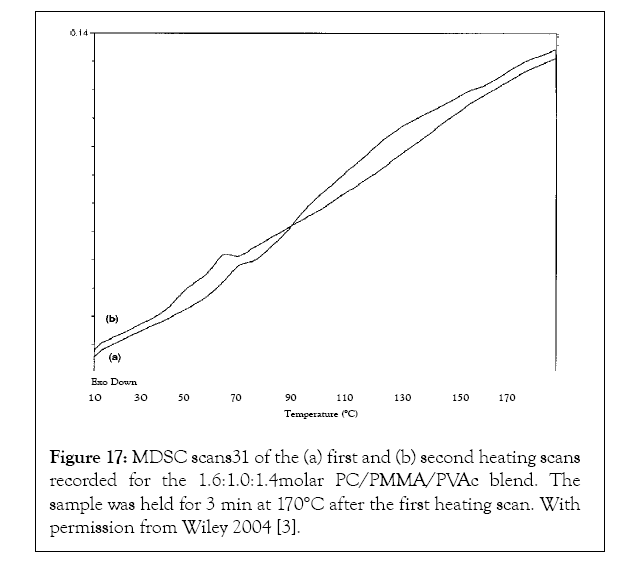
Figure 17: MDSC scans31 of the (a) first and (b) second heating scans recorded for the 1.6:1.0:1.4molar PC/PMMA/PVAc blend. The sample was held for 3 min at 170°C after the first heating scan. With permission from Wiley 2004 [3].
Figure 18 presents the TGA scans of co-precipitated and coalesced PC/PMMA/ PVAc blends. The phase segregated coprecipitated blend shows three distinct degradation events, one for each polymer component. Consistent with its well-mixed character, the coalesced PC/PMMA/PVAc blend shows a single broad thermal decomposition. Specific interactions between component polymer chains in the molecularly mixed coalesced ternary blend, that are absent in the phase segregated coprecipitated blend, are likely the source for the significant difference in their thermal decomposition behaviors. Both restructured isotactic (i)-PP and isotactic-poly (1-butene) (i-PB) samples have been obtained via coalescence for their individual γ-CD-ICs [44]. c-i-PP crystallized at a higher temperature and to a greater extent from its melt than an as-received (asr)-i-PP melt sample.
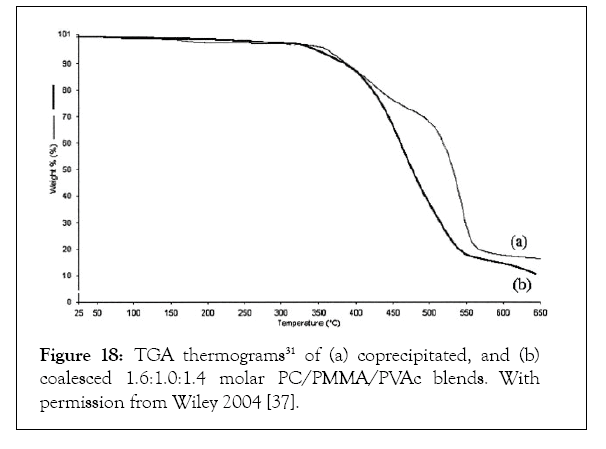
Figure 18: TGA thermograms31 of (a) coprecipitated, and (b) coalesced 1.6:1.0:1.4 molar PC/PMMA/PVAc blends. With permission from Wiley 2004 [37].
While i-PP can be crystalized in several different polymorphs, the i-PP chains adopt the typical 31 helical conformations in each. On the other hand, i-PB can, depending on the preparation method, crystallize in five different crystal phases, which differ in helical conformations and chain packing [45-56]. Form III polymorph (orthorhombic unit cell and a 41 helical chain conformation), can be obtained by crystallizing from various solvents. Crystallization from the melt under high pressure produces the form I/and form II/polymorphs. Depending on the solvent, concentration, and crystallization temperature, forms I/and III can also be obtained from solution crystallization.
Commonly obtained by melt crystallization at room temperature and without constraints, the Form II polymorph (tetragonal unit cell and a 113 helical conformation), and transforms slowly and irreversibly to the most stable form I polymorph (hexagonal unit cell and 31 helical conformation). On the other hand, c-i-PB crystallized into its form II polymorph, rather than the usual more stable form I polymorph obtained from asr-i-PB. Furthermore, form II c-i-PB transformed much more slowly into the more stable form I than typically does the form II polymorph of asr-i-PB. Form II c-i-PB made with an asr-i-PB having a molecular weight of 380,000 remained stable more than 6 months, while asr-i-PB form II crystals are observed to convert to the more stable form I polymorph with half-lives of ~4 to 24 hr [53]. It should be mentioned that the transition from form II to form I i-PB polymorphs can be shortened by an order of magnitude if the form-II sample is rotated at a speed of 2,400 MHz [57].
In addition, i-PP/i-PB blends were also obtained via coalescence from their common γ-CD-IC [58]. The c-i-PP chains in the i-PP/ i-PB blends crystallize as they do in neat c-i-PP. The i-PB chains in the c-i-PP/i-PB blends crystallize much faster than neat asr-i- PB and as fast as asr-i-PB chains do from solution. Unlike neat coalesced i-PB samples, the i-PB chains in coalesced i-PP/i-PB blends initially crystallizes in Forms I’ and III polymorphs, which gradually transform into Form II crystals upon heating. In contrast, the phase separated blend samples made from solutions containing both i-PP and i-PB show crystallization behaviors similar to neat i-PP and neat i-PB samples.
It is thus apparent that the crystallization behaviours of both i-PP and i-PB chains from their melts is affected by coalescence and their intimate blending in c-i-PP/i-PB samples. This suggests that the study of both crystalline coalesced neat polymer A and polymer B samples and their comparison to the crystallization of polymer A/polymer B bend samples formed by their coalescence from common CD-ICs may provide important information relevant to the mechanisms of polymer crystallization.
Blends coalesced from their CD-ICs, with the exception of coalesced PS/PC and PET/PEN blends [35,59], remained intimately mixed even after annealing above their Tgs and Tms for substantial periods (30 min or longer). The coalesced PS/PC blend did in fact eventually segregate into two phases with distinct Tgs close to those of pure PS and pure PC, but only after multiple heating/cooling DSC cycles [35]. On the other hand, the partially well-mixed coalesced PET/PEN blends rapidly transesterified into PET-PEN copolymers [35].
The intimacy of mixing of all other common CD-IC processed homopolymer blends were observed not to be affected by rather prolonged heating at high temperatures. This even though both component homopolymers should have possessed sufficient mobility to phase segregate under the thermodynamic driving force provided by their inherent incompatibility, that is, Δ Hmix>0.
The configurational/mixing entropies of their coalesced and initially intimately mixed blends, though presumably small for pairs of high molecular weight polymers [27,59], oppose their unfavorable heats of mixing and somewhat hinder their phase segregation. The net (enthalpic–entropic) driving force for segregation of these CD-processed and initially intimately mixed, yet normally incompatible, homopolymer pairs, is not sufficient to significantly speed up the inherently slow/sluggish center-of-mass movements expected of bulk polymer chains that are required to achieve separate phases [15,29]. Their slow center-of-mass mutual diffusion likely controls their phase segregation [27].
Because Tgs intermediate between those of the neat blend components are observed in at least their first several DSC heating scans, the final and presumably slowest step in the phase segregation of initially well-mixed coalesced blends s in (a) Figure 19, formed between inherently incompatible polymers, likely proceeds from the well-mixed randomly coiling, yet not fully entangled blends in (b). The initially coalesced blend (a), which, with its largely extended, non-coiled chains, that might not be expected to show a Tg at all, apparently rapidly converts to (b), with a glass transition characteristic of mixed randomly coiling blends. Thus, the considerable thermal and temporal stabilities of wellmixed blends obtained by coalescence from their common CD-ICs can most likely be attributed to the long-lived metastability of their well-mixed randomly-coiling states (b), where, however their chains may not sufficiently interpenetrate and fully entangle.
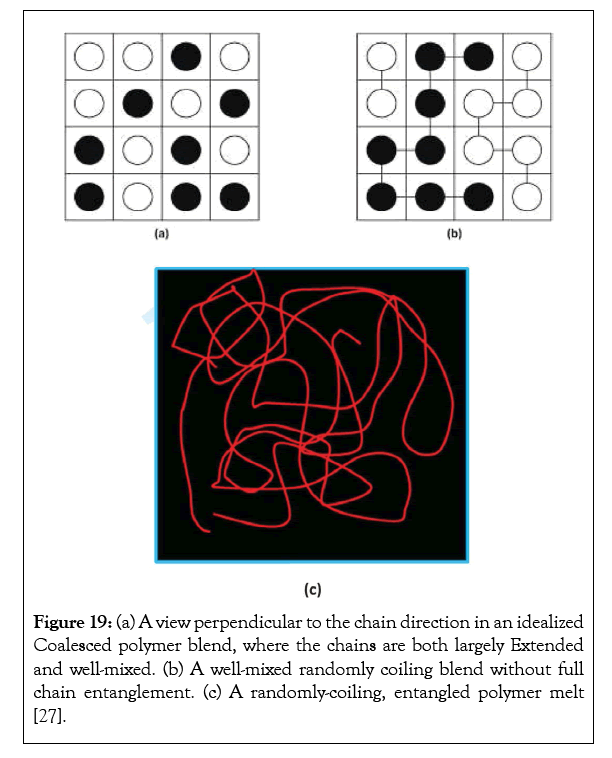
Figure 19: (a) A view perpendicular to the chain direction in an idealized Coalesced polymer blend, where the chains are both largely Extended and well-mixed. (b) A well-mixed randomly coiling blend without full chain entanglement. (c) A randomly-coiling, entangled polymer melt [27].
Potential applications for single polymer composites and miscible blends
To begin with, the single polymer composites and miscible blends produced from their ICs contain are completely pure materials containing no additives. As a consequence, they are likely more easily recycled, and if biocompatible maybe used in in vivo medical applications. Furthermore, their composite and blended films and fibers are stronger than those made from the same asr-polymers, and their material behaviors may also be readily tunable.
For example, the degradation of miscible blends made from the coalescence of common ICs containing a mechanically strong stable polymer and an easily degradable polymer suggest a means for reducing the accumulation of polymer waste. Because the same c-and asr-polymers are miscible, self-nucleation is possible for single polymer composites that are ground up, melted, and cooled.
Intimately mixed blends obtained by the coalescence of two ordinarily immiscible polymers from their common ICs may yield materials with unique properties.
In general, it appears that single polymer composites and well mixed blends can be obtained via coalescence from their CDICs. The resulting composites and blends have improved physical properties and are stable to prolonged heating above their Tgs and Tms. Consequently, these CD-IC processed polymer materials have many potential unique applications.
[Cross Ref] [Google Scholar] (All Versions)
[Cross Ref] [Google Scholar] (All Versions)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Versions) [PubMed]
[Google Scholar] (All Versions)
[Cross Ref] [Google Scholar] (All Versions)
[Cross Ref] [Google Scholar] (All Versions) [PubMed]
[Cross Ref] [Google Scholar] (All Versions) [PubMed]
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version) [PubMed]
[Cross Ref] [Google Scholar] (All Version) [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version) [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version) [PubMed]
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version) [PubMed]
[Cross Ref] [Google Scholar] (All Version) [PubMed]
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
[Cross Ref] [Google Scholar] (All Version)
Citation: Tonelli AE (2022) Production of Single Polymer Composites and Miscible Polymer Blends via Nano-restructuring: Cyclodextrin and Urea Inclusion Compounds. J Nanomedine Biotherapeutic Discov 12:174.
Received: 02-Jun-2022, Manuscript No. JNBD-22-17710; Editor assigned: 06-Jun-2022, Pre QC No. JNBD-22-17710; Reviewed: 21-Jun-2022, QC No. JNBD-22-17710; Revised: 29-Jun-2022, Manuscript No. JNBD-22-17710; Accepted: 13-Jul-2022 Published: 20-Jul-2022 , DOI: 10.4172/2155-983X. 22.12.174
Copyright: © 2022 Tonelli AE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.